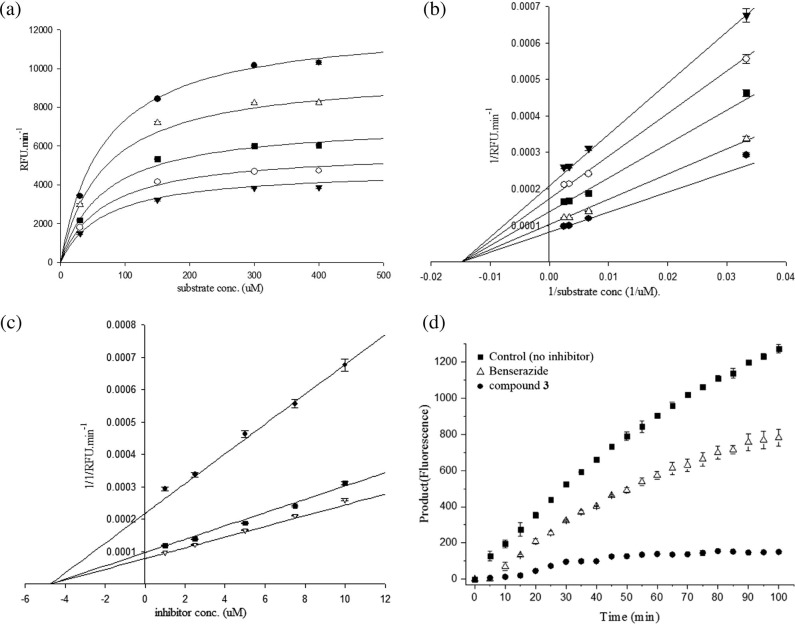
Fig. 2.
Inhibitory mechanism study of 4 (Benserazide) by enzyme kinetic and reversibility studies. (a) Michaelis–Menten plot. In the presence of various concentrations of substrate and inhibitor, CVB3 3Cpro activity was measured by fluorescence. The x-axis is the substrate concentration, and the у-axis is the CVB3 3Cpro activity (RFU min−1). Each plot describes different inhibitor concentrations 0 μM (●), 2.5 μM (△), 5.0 μM (■), 7.5 μM (○), and 10 μM (▾). (b) In the Lineweaver–Burk plot, the Benserazide (4) concentration was set to 0 μM (●), 2.5 μM (△), 5.0 μM (■), 7.5 μM (○), and 10 μM (▾). The x-axis is 1/substrate concentration and the у-axis is the 1/CVB3 3Cpro activity (1/RFU min−1). (c) In the Dixon plot, the substrate concentration was set to 30 μM (●), 150 μM (■), and 300 μM (▽). The x-axis is the inhibitor concentration, and the у-axis is the 1/CVB3 3Cpro activity (1/RFU min−1). (d) The reversibility plot, the x-axis is time and the у-axis is product (fluorescence). Benserazide concentration (△) was set 2.5 μM and compound 3 concentration (●) was set 150 nM.