Abstract
A bi-cistronic baculovirus expression vector was constructed to facilitate the expression, detection, and isolation of the hemagglutinin (HA) fragment HA1 of H6N1 avian influenza virus (AIV) in an insect and a culture of its cells. In this construct, the GP67sp signal peptide promoted the secretion of the recombinant protein into the culture medium, and improved protein expression and purification. Enhanced green fluorescent protein, co-expressed through an internal ribosome entry site, served as a visible reporter for protein expression detection. The hemolymph of Spodoptera litura larvae infected with the bi-cistronic baculovirus was collected for the purification of the recombinant HA1, which was found to be glycosylated, and monomeric and trimeric forms of the recombinant HA1 were identified. Proteins expressed in both the cell culture and larvae served as effective subunit vaccines for the production of antiserum against HA. The antiserum recognized the H6 subtype of AIV but not the H5 subtype.
Keywords: Avian influenza virus, Bi-cistronic baculovirus expression system, Hemagglutinin, Spodoptera litura larvae
Abbreviations: AIV, avian influenza virus; EGFP, enhanced green fluorescent protein; HA, hemagglutinin; IRES, internal ribosome entry site; NA, neuraminidase; PPH, polyhedrin promotor; RBD, receptor binding domain
Highlights
-
•
HA1 of H6N1 influenza virus was expressed in insect and cell culture.
-
•
The expressed HA1 was glycosylated, and estimated as monomeric and trimeric forms.
-
•
The expressed HA1 served as an effective subunit vaccine for producing antisera.
-
•
The antisera specifically recognized influenza H6 subtype but not the H5 subtype.
1. Introduction
In the last decade, avian influenza virus (AIV) has provoked seasonal epidemics and caused severely infectious respiratory disease (Palache et al., 2015). AIV is a member of the Ortho-myxoviridae family and involves two major surface glycoproteins: hemagglutinin (HA) and neuraminidase (NA) (Gamblin and Skehel, 2010; Zhu et al., 2013). On the surface of influenza A virus, 18 subtypes of HA, and 11 subtypes of NA are present (Mehle, 2014). HA is a lectin that mediates binding of virus and the sialic acid receptor on the host cell membrane, facilitating entry of the viral genome into the host (Skehel and Wiley, 2000). A tryptic cleavage in the molecule of HA produces HA1 and HA2 fragments. This post-translational cleavage, formed by trypsin, activates the receptor binding domain (RBD) on HA1 so that it may bind to the host receptor. HA is a vital antigen, but it is inclined to mutate to escape the attack from the immune system (Van de Sandt et al., 2012). Glycosylation on HA may play a critical role in triggering an immune response (Wang et al., 2009; He et al., 2015). Traditional human inactivated vaccines for influenza are produced by using the specific-pathogen-free (SPF) embryonated egg method, which requires specialized equipment and complex procedures (Nunez et al., 2015). However, some people are allergic to eggs, and inoculation with these vaccines involves risk (Franceschini et al., 2015). A subunit vaccine for AIV produced using a recombinant protein expression system may serve as an alternative.
Autographa californica nucleopolyhedrovirus (AcMNPV) is a baculovirus with double-stranded DNA that infects some Lepidoptera Noctuidae insects, but it does not infect humans. AcMNPV has been utilized in biopesticides and as a vector for foreign gene expression to produce recombinant proteins (Kost et al., 2005). Yang et al. (2007) constructed a pseudotyped baculovirus that expressed HA on the viral surface and showed vaccine potential. Musthaq et al. (2014) expressed HA of the H6 subtype of AIV and displayed it on the baculovirus envelope, demonstrating its protective efficacy for mice against H6 influenza virus. However, in these methods, production of unrelated antibodies against several indigenous proteins from baculovirus was cause for concern. The production and use of a purified subunit vaccine should be straightforward and convenient. The protein expression level in insect cells is relatively low, and the target protein may be cleaved by proteases released from the cells. If the recombinant protein secretes into a culture medium, and a visible reporter may be introduced into the construct to serve as an indicator for protein expression, facilitating the determination of the precise time for cell harvest.
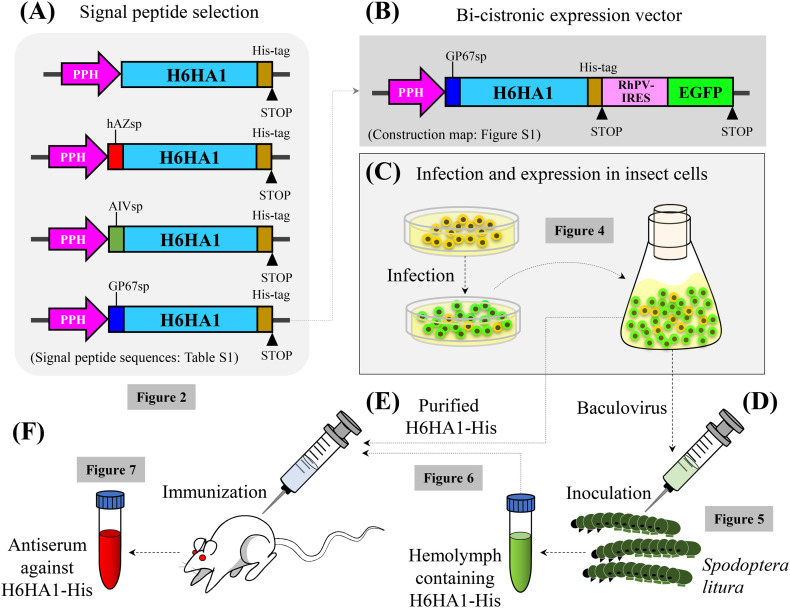
In addition, multiple promoters may compete for RNA polymerase in gene transcription (Hu et al., 2006). Therefore, we designed a dual expression system led by single promoter for the co-expression of the target H6 subtype HA1 protein (H6HA1-His) and the reporter (enhanced green fluorescent protein, EGFP). An internal ribosome entry site (IRES) was applied to translate EGFP by starting its initiation in the middle of mRNA (Finkelstein et al., 1999; Chen et al., 2005; Wu et al., 2012). For the production of the secreted recombinant HA1 to facilitate purification, we first examined the expression and secretion efficiency for H6HA1-His in three signal peptides. Among these signal peptides, GP67sp has been used for its expression of HA in a baculovirus system (Whitford et al., 1989; Cui et al., 2011). After successful expression in insect cells, the vector was used to infect insect larvae, which served as bioreactors. Bombyx mori nucleopolyhedrovirus (BmNPV) and silkworm systems have been reported to exhibit high protein expression levels (Katsuma et al., 2006; Kato et al., 2010; Kajikawa, 2012). As an alternative, we developed an AcMNPV expression platform (Choi et al., 2012) in Spodoptera litura (cotton leafworm or tobacco cutworm), which is often used in academic research and is easy to breed and manage. The expression and purification of the H6HA1-His recombinant protein in the insect cells and larvae, the protein structure and modification of the expressed proteins, and the use of the proteins for immunization in mice are discussed in this report and are summarized in Fig. 1 .
Fig. 1.
Schematic flow chart outlining this study.
This chart summarizes the construction of the bi-cistronic expression system (A and B), its application in infecting insect cells (C) or larvae (D), and the expression and isolation of the H6HA1-His recombinant protein (E). The purified proteins were further used to immunized mice (F). A detailed map of the construction of the pFast-GP67-H6HA1-His-RhPV-IRES-EGFP vector is presented in Supplementary Fig. S1.
2. Materials and methods
2.1. Construction of the bi-cistronic baculovirus expression vector
The H6HA1-His coding sequence derived from the plasmid pET-32b-His-H6HA1-His (He et al., 2014) was amplified using conventional polymerase chain reaction (PCR) procedures and subcloned into the pFastBac1 baculovirus expression vector by using TA cloning (Zhou et al., 1995) (Supplementary Fig. S1). The coding sequence of the signal peptides used in this work (i.e. AIVsp, hAZsp, and GP67sp) was ligated upstream of the H6HA1-His coding sequence by overhang extension PCR (Kadkhodaei et al., 2016). The polyhedrin promotor gene of AcMNPV (PPH) was used to drive the H6HA1-His recombinant protein (Possee and Howard, 1987). Subsequently, the RhPV-IRES derived from Rhopalosiphum padi virus (Wu et al., 2012), followed by the EGFP reporter gene, was ligated to the downstream of the GP67-H6HA1-His coding sequence. The final pFastBac-GP67-H6HA1-His-RhPV-IRES-EGFP construct was transferred to DH10Bac competent cells (Thermo Fisher Scientific) and the recombinant bacmid was produced according to the manufacturer's instructions. Four potential N-linked glycosylation sites on H6HA1-His (Asn-11, Asn-23, Asn-167, and Asn-291) were mutated to alanine through PCR-based site-directed mutagenesis (He et al., 2015).
2.2. Insect cell culture
Spodoptera frugiperda Sf21 cells were cultured at 28 °C in TNM-FH medium (Sigma, USA) containing 10% fetal bovine serum for the generation of recombinant baculovirus (Chen et al., 2005). For protein expression, Sf21 cells were cultured at 28 °C in Sf-900II serum-free medium (Gibco, USA).
2.3. Generation of the recombinant baculovirus
Sf21 cells (2 × 105 cells) in 24-well plates were transfected with the recombinant bacmid containing the coding sequence of GP67-H6HA1-His-RhPV-IRES-EGFP (3 μg) by using 3 μL of Cellfectin (Invitrogen) for generation of the recombinant vAcGP67-H6HA1-His-RhPV-IRES-EGFP baculovirus. At day 4 after the transfection, the 24-well plates were screened with an inverted fluorescence microscope to analyze the intensity of the EGFP fluorescence and to identify cells with H6HA1 expression. The titer of the recombinant baculovirus was determined with end-point dilution according to the 50% tissue culture infectious dose (TCID50) method in a 96-well plate. Sf21 cells (2 × 105 cells) cultured in 24-well plates were then infected with vAcGP67-H6HA1-His-RhPV-IRES-EGFP baculovirus at a multiplicity of infection (MOI) of 1. The baculovirus supernatant was collected and centrifuged to remove cell debris. It was then passed through a 0.22 μm syringe filter before being stored at 4 °C.
2.4. Expression and purification of the recombinant H6HA1-His proteins
Sf21 (2 × 107 cells) were seeded in a T75 flask and cultured at 28 °C in Sf-900II serum-free medium. The cells were then infected with vAcGP67-H6HA1-His-RhPV-IRES-EGFP baculovirus at an MOI of 1. The culture medium was collected by centrifugation after 72 h of infection. The protein in the culture medium was precipitated using ammonium sulfate fractionation at 80% saturation, and the pellet was resuspended in 10 mL phosphate buffered saline (PBS) buffer. After centrifugation, the clear supernatant was mixed completely with His-select nickel affinity gel (BIOMAN, Taiwan) overnight. The resin was then loaded into a column by gravity flow and was washed with 20 gel volumes of washing buffer (50 mM Tris-HCl, 300 mM NaCl, 10% glycerol and 20 mM imidazole, pH 7.4). The recombinant H6HA1-His protein was then eluted with elution buffer (same as washing buffer except for the inclusion of 200 mM imidazole). All steps of protein purification were performed at 4 °C.
2.5. Mouse immunization
Female BALB/c mice (6 weeks old) were purchased from the National Laboratory Animal Center, Taiwan, and three mice were used per inoculation group. For every inoculation, 100 μg recombinant H6HA1-His protein was emulsified with equal volume of Freund's complete or incomplete adjuvant (Sigma, USA). During the 10-week immunization schedule (five booster shoots at every 2-week interval), mouse sera were collected and analyzed for specificity using Western blotting against antigens including the expressed H6HA1-His proteins from Escherichia coli or baculovirus, and two subtypes of AIV (the H6 subtype [A/chicken/Taiwan/2838 V/00] and the H5 subtype [A/Duck/Taiwan/3233/04]), which were provided by Professor Ching-Ho Wang (Department of Veterinary Medicine, National Taiwan University, Taiwan). These animal experiments were approved by the Institutional Animal Care and Use Committee of National Taiwan University.
2.6. Infection and expression of baculovirus in the larvae of S. litura
The larvae of S. litura were provided by Dr. Yue-Long Wu (Department of Entomology, National Taiwan University, Taiwan). They were fed an artificial diet and raised in a 27 °C environment (Tuan et al., 2014). A virus solution of vAcGP67-H6HA1-His-RhPV-IRES-EGFP (100 μL, 2 × 108 pfu/mL) was injected into the midgut of each larva at its final instar stage (Rivkin et al., 2006). The larvae that were infected with baculovirus became swollen in appearance within 5 days. These larvae were sacrificed using CO2, and the tails of the larvae were cut off to facilitate hemolymph collection. The supernatant of the hemolymph was purified by ammonium sulfate fractionation (60%–80% saturation), followed by gel filtration (Superdex 75 pg, GE Healthcare Life Sciences) and nickel affinity chromatography, as already described (see 2.4).
3. Results
3.1. Signal peptide GP67sp facilitated the expression and secretion of H6HA1-His recombinant protein in insect cells
To increase the expression and secretion of the H6HA1-His protein in baculovirus, three secretory signal peptides were separately added to the upstream section of H6HA1-His sequence (Fig. 1A). Supplementary Table S1 presented these signal peptides (hAZsp, AIVsp, and GP67sp) and their amino acid sequences. The recombinant baculoviruses carrying the H6HA1-His genes with the various secretory signal peptides were used to infect Sf21 cells in serum-free culture. Subsequently, the culture medium was collected for the Western blot analysis using an antibody against HA1 (Anti-HA). As illustrated in Fig. 2A, GP67sp most efficiently expressed the H6HA1-His protein in the medium compared with hAZsp and AIVsp under the same conditions. H6HA1 without an N-terminal signal peptide could not be detected in the medium (control in Fig. 2A). After Western blot analysis, the H6HA1-His protein was purified using a nickel affinity column, and then eluted using stepwise imidazole concentrations. GP67sp was chosen for the secretion of H6HA1-His in the culture media of the following experiments.
Fig. 2.
Comparison of signal peptides on the expression of H6HA1-His.
Three signal peptides were added respectively to the upstream of the H6HA1-His sequence to enhance the secretion of the H6HA1-His protein. These peptides (hAZsp, AIVsp, and GP67sp) and their amino acid sequences are presented in Supplementary Table S1. (A) The expressed proteins in the medium of the cell cultures were collected by ammonium sulfate precipitation (AS), and then nickel affinity chromatography was applied. After the unbound proteins (Ub) were washed out, the H6HA1-His proteins were eluted from the column using imidazole gradients that were detected in Western blotting using an anti-HA antibody. All experiments were performed under the same conditions for comparison. (B) The expressed H6HA1-His proteins from the cell pellet (Cell) or the supernatant (Sup) were compared using Western blotting, for which their protein amount was adjusted to be equal. No signal peptide was added in the control experiments.
Fig. 2B presents a comparison of the expression of H6HA1-His proteins in the cell (Cell) and the medium (Sup), in which the protein quantities of the samples were adjusted to be equal. The results indicated that the three signal peptides demonstrated similar efficiency in bringing the H6HA1-His protein into the medium. By contrast, no H6HA1-His was detected in the supernatant of the control in which no signal peptide had been added, indicating that the PPH promotor alone was not sufficient for protein secretion.
3.2. Expressed H6HA1-His protein was glycosylated in insect cells
The molecular mass of the expressed H6HA1-His was estimated as 40 kDa in the Western blots (Fig. 3A, WT), which was larger than that expected from the amino acid sequence (38 kDa), implying that the expressed H6HA1-His from the insect cells may have been glycosylated. This was confirmed by the finding that if one of the glycosylated sites on HA1 (Asn 167) was mutated to Ala (N167A), the molecular mass of HA1 decreased. Additionally, when all four glycosylation sites on HA1 were mutated (i.e. N11A, N23A, N167A, and N291A), the mutant exhibited an even smaller molecular mass of 38 kDa (Fig. 3A, lane M4). Furthermore, if the recombinant protein was treated with peptide-N-glycosidase F (PNGase-F) to remove the glycans, its molecular mass decreased to that of the mutant M4 (Fig. 3B). The band intensities of the modified H6HA1 proteins were also substantially reduced, because they were more sensitive to proteolytic attack in the absence of protection from glycans. A schematic of the glycosylation of HA1 presented in Fig. 3C. A previous study reported that the glycan in insect cells comprised six monosaccharides, and exhibited a molecular mass of approximately 1.1 kDa (Shi and Jarvis, 2007). However, the glycan of HA on AIV produced in mammalian cells comprised 12 monosaccharides, and exhibited a molecular mass of approximately 2.5 kDa (Shi and Jarvis, 2007) (Supplementary Fig. S2). In this study, we did not analyze the sugar composition of the glycan on the expressed H6HA1-His protein.
Fig. 3.
Glycosylation of the expressed H6HA1-His.
The molecular mass of the expressed H6HA1-His decreased when the glycosylation sites were mutated. (A) Mutants in which one glycosylation site (N167A) or all four sites (M4) were deleted expressed H6HA1-His proteins with smaller molecular mass. (B) The expressed protein was treated with glycosidase (PNGase-F) to remove the glycan; both molecular mass and band intensity decreased. (C) A schematic showing four possible glycosylation sites on H6HA1.
3.3. Expression of the bi-cistronic recombinant baculovirus in insect cells
To facilitate recombinant H6HA1-His protein production, a secretory bi-cistronic recombinant baculovirus was constructed to co-express the H6HA1-His recombinant protein with EGFP (Fig. 1B). These two genes were transcribed by the same PPH promotor in the same transcript, and their translations were controlled by cap and RhPV-IRES, respectively. To optimize recombinant H6HA1-His protein production, two insect cell lines, Sf21 (Fig. 4 ) and High Five (Supplementary Fig. S3), were tested for baculovirus infection. The images in Fig. 4A indicate that EGFP may serve as a visible selection marker for baculovirus infection and may be used to monitor the expression in the infected insect cells. As shown in Fig. 4B, when a cell lysate containing the expressed H6HA1-His protein was purified on a nickel column, it was contaminated by EGFP due to non-specific adsorption. By contrast, the culture medium exhibited a higher concentration of the expressed protein, which was separated clearly from the EGFP using an affinity column (Fig. 4C). An inconsistency between the HA1 amounts (imidazole elutions) determined by anti-HA and anti-His antibodies (Fig. 4B and C) was identified. The structural environment in the expressed proteins may have caused the instability in the His tags, resulting in this inconsistency.
Fig. 4.
Expression of bi-cistronic recombinant baculovirus in Sf-21 cell.
The bi-cistronic recombinant baculovirus was used to infect the insect cell Sf-21. (A) The reporter EGFP displayed fluorescence in the cell culture (right panel; bright-field image at left panel); this signal might serve as a visible indicator of appropriate cell harvest time. (B) The cells were collected at 72 h and disrupted to obtain the crude extract (XT), which was precipitated for the crude protein (CP), and then applied to the nickel column. After the unbound proteins were washed out (Ub), the H6HA1-His proteins were eluted with imidazole gradient. (C) The culture medium was collected and precipitated by ammonium sulfate (AS); it was then dissolved in buffer and then applied to the nickel column and eluted with imidazole. (D) The culture medium was collected at scheduled time intervals after infection, and proteins were subsequently revealed by Western blotting.
The results presented in Fig. 4D indicated that the expression of H6HA1-His started 48 h after infection and increased until up to 144 h after infection, and the expression of EGFP was 24 h behind. Thus, H6HA1-His was harvested from the culture medium as soon as green fluorescence was observed in the cells, approximately 72 h after infection. Sf21 and High Five cells exhibited similar results for baculovirus expression (Fig. 4 and Supplementary Fig. S3).
3.4. Production of recombinant H6HA1-His protein in S. litura larvae
The bi-cistronic baculovirus was further used to infect S. litura larvae to demonstrate the use of the insect as a bioreactor. Because this recombinant protein contained GP67sp, the expressed H6HA1-His was expected to be secreted into the hemolymph (Maeda et al., 1985), from which it could be isolated and purified for further applications. We inoculated 60 larvae; of these, 42 survived, and we collected their hemolymph for Western blot analysis (Fig. 5 ). Half of the surviving larvae (21) produced the recombinant proteins, as revealed by antibodies against HA (Fig. 5B) and His (Fig. 5C). Some larvae exhibited relatively high concentrations of the expressed protein (e.g., larvae no. 3, 5, 19, 35, and 37). The hemolymph from all 21 larvae with protein expression were collected and mixed to a final volume of 500 μL.
Fig. 5.
Expression of the recombinant baculovirus in S. litura larvae.
S. litura larvae were inoculated with the recombinant baculovirus. (A) Within 1 week, the infected larva had swollen. (B) Sixty larvae were inoculated, and 42 survived. Larval hemolymph was collected, and H6HA1-His was detected by Western blotting using anti-HA (B) or anti-His (C). The H6HA1-His protein from the infected Sf-21 cell was used as the positive control (P), and the normal hemolymph from the blank larva was used as the negative control (N).
The entire collected hemolymph (500 μL) was fractionated with ammonium sulfate (Supplementary Fig. S4), and fractions between 60% and 80% saturation were pooled for further purification by gel filtration with Superdex 75 pg (Fig. 6A). The eluted fractions were analyzed through Western blotting to reveal H6HA1-His protein (Fig. 6B). Two major distributions were observed around fractions 34 and 42, and their native molecular masses were estimated as 94 and 35 kDa, respectively. We speculated that the first peak was the dimeric or trimeric form of the H6HA1-His protein, and the 35 kDa peak was the monomer. These samples were further purified to near homogeneity by using a nickel affinity column (Supplementary Fig. S5). The concentration of the H6HA1-His protein in the hemolymph was estimated as 450 μg/mL by comparing Coomassie Blue band intensities on a gel of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of the H6HA1-His protein in the cell culture medium was estimated as 5 μg/mL.
Fig. 6.
Gel filtration analysis of the H6HA1-His protein from hemolymph.
(A) After ammonium sulfate fractionation (Supplementary Fig. S4), the proteins were separated by gel filtration, which revealed one large protein peak and several minor peaks (blue curve). Three standards (a, b, and c) were used as molecular weight markers, and they were applied under the same conditions (orange curve). (B) Selected fractions were analyzed using SDS-PAGE and then Western blotting to reveal H6HA1-His proteins. CBR, Coomassie Blue R staining. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Mouse immunization with the H6HA1-His recombinant proteins
BALB/c mice were immunized using purified H6HA1-His from insect cells and hemolymph. After several booster shoots, the antisera from the mice showed sufficient titer levels at 4000-dilution against the HA1 recombinant protein expressed in E. coli (Fig. 7A). The antisera were further examined for specificity against antigens from various sources. In Fig. 7B, HA1 proteins from insect cells (lane a) and E. coli (lane b) are compared. The band from E. coli (lane b) exhibited a higher molecular-mass (55 kDa) due to an extra thioredoxin tag and doubled His-tags in the construct. Numerous lower-molecular-mass bands were also exhibited, and these may have been caused by proteases in the cell lysate. In addition, two subtypes of AIV (H5 and H6) were compared for antigen specificity (lanes c-f). The antiserum was not found to recognize any protein from the H5 subtypes (lanes e and f). However, it did reveal bands of H6 subtypes with a molecular mass of approximately 44 kDa (lanes c and d). This result indicates that this antiserum was specific to the H6 subtype of HA.
Fig. 7.
Expressed H6HA1-His proteins were effective antigens.
Isolated H6HA1-His proteins were used as antigens to immunize mice for the production of the antisera. (A) During the 10-week immunization schedule, antisera against recombinant proteins from either insect cells (A2) or hemolymph (A3) revealed H6HA1-His bands in Western blots at a 4000-dilution titer. A control experiment was performed in which mice were immunized mice with PBS (A1). (B) The antiserum at the fifth booster shoot was further used to detect antigens from various sources: H6HA1-His isolated from insect cells (a) or E. coli (b); H6HA1 viral protein from the H6 subtype of AIV (c and d); and the H5 subtype (e and f). Higher protein concentration was applied in lanes d and f.
4. Discussion and conclusion
In this study, we constructed a secretory bi-cistronic baculovirus expression system in insect cells. The system produced the influenza H6HA1-His recombinant protein, and EGFP was used as a visible reporter (Fig. 1B). The signal peptide GP67sp facilitated the secretion of H6HA1-His into a medium of insect cell culture, which was then purified using a nickel column (Fig. 2). The H6HA1-His protein produced was determined to be glycosylated based on its molecular mass as indicated by SDS-PAGE (Fig. 3A). SDS-PAGE analysis showed that the protein glycosylation in this expression system was relatively complete in terms of glycan sites. We estimated that each expressed HA1 protein involved three to four glycans, and each glycan exhibited a molecular mass of approximately 1 kDa. Although these glycans were produced in insect cells rather than mammalian cells, the expressed HA1 protein served as an effective subunit vaccine for immunizing mice. The mice immunized with the vaccine generated relatively high-titer antisera against the virus prepared from embryonated SPF eggs (Fig. 7). The antisera in the mice were specific to the H6 subtype of AIV but did not recognize the H5 subtype. However, the recombinant HA1 exhibited no hemagglutination activity (data not shown), which may have been due to the absence of the HA2 domain in complete HA. The baculovirus was also used to infect S. litura larvae (Fig. 5). Hemolymph from the infected larvae was collected, and the expressed protein was purified using a general purification procedure. On the gel filtration elution pattern, the native H6HA1-His protein was expressed as a monomer with a molecular mass of 35 kDa, along with a high-molecular-mass polymeric form (Fig. 6A). On the basis of the distribution of the bands revealed by anti-HA antibody on the Western blot (Fig. 6B), we speculated that the polymeric form may have been a trimer. The earliest band was eluted at fraction number 33, corresponding to a molecular mass of approximately 100 kDa, nearly three-fold that of the monomer (35 kDa). Previous studies reported that the native HA on the viral particle comprises three identical monomers (Gamblin and Skehel, 2010; Zhu et al., 2013), and may show a modulation of the monomer-trimer equilibrium (Seok et al., 2017). In this study, although the expressed protein contained only the HA1 fragment of HA, the expression of this fragment was sufficient for formation of a trimer. The molecular mass of the monomer estimated using gel filtration (35 kDa) was smaller than that estimated using SDS-PAGE (40 kDa). Accordingly, we considered SDS-PAGE to be more precise than gel filtration for determining the subunit molecular mass of the protein.
On the basis of the study results, we concluded that the proposed secretory bi-cistronic baculovirus expression system is a useful and efficient platform for stimulating the expressing of recombinant HA1 in insect cells and larvae. In the experiment, a large proportion of the expressed protein was secreted into the culture medium and could be harvested directly, avoiding contamination from cytosolic proteases. The H6HA1-His produced in E. coli exhibited extensive degradation (Fig. 7B, lane b) compared with that from the insect cells (Fig. 7B, lane a). In addition, the visible reporter EGFP indicated the appropriate time for cell harvest, ensuring that harvest was performed at the time of highest expression of the recombinant protein and minimizing the disruption of host cells. In this study, the cells were harvested 3 days after infection. The expressed viral protein was glycosylated and was assembled in a trimeric form (Fig. 6) similar to that of the native viral protein. This similarity might explain why the expressed H6HA1-His protein served as an effective subunit vaccine (Fig. 7). A previous study indicated that glycosylation in a baculovirus expression system was not as effective as that in mammal cells, but that the protein folding and expression level were equal to or better than those from the prokaryotic system (Harrison and Jarvis, 2006). The baculovirus expression system presented in this study may serve as an effective vector to construct virus-like particles for vaccine production. Although this system expressed H6HA1-His protein and secreted into the medium efficiently, we found that it was not successful in producing the spike protein (S1) of Middle East respiratory syndrome coronavirus (Du et al., 2017). The secretion of S1 was poor, even though it was expressed at adequate level in the cell (data not shown). The system should be used to test all proteins individually for their expression and secretion by using proper promotor or signal peptides.
The system produced the H6HA1-His protein efficiently in the medium of insect cell culture. However, the cell culture is expensive and the procedure is time consuming. We attempted to stimulate the expression of the recombinant baculovirus protein in its natural host, and we found that the S. litura larvae were suitable bioreactors for this purpose (Fig. 5). S. litura is an agricultural pest, and a large population is easy to raise for the collection of hemolymph. However, hemolymph is a complex mixture of biological molecules that may contaminate the expressed protein. Purification was required to isolate the expressed protein; in this study, ammonium sulfate fractionation, gel filtration, and a nickel affinity column were used for this purification process. In future research, we will replace the His-tag with another type of affinity tag, because the hemocyanin in hemolymph may bind to the nickel column and interfere with the affinity purification of the H6HA1-His protein. Additionally, this construct was not protected by the polyhedral baculovirus protein, feeding could not be used to infect larvae. Instead, we manually injected the baculovirus into the midgut of the larvae. The survival rate of the larvae after the injection was only 70%, and half of the surviving larvae produced the recombinant protein. Use of an auto-injection device may result in a high proportion of survival. An additional problem was caused by use of the S. litura larvae rather that B. mori larvae; the fluorescence of the expressed EGFP on the larvae of S. litura could not be observed, because the skin of the larva is too dark. However, the larvae infected with baculovirus became observable swollen (Fig. 5A). Therefore, visual examination was sufficient to identify the larvae in which the proteins were expressed in adequate amounts.
In conclusion, the presented system is efficient for H6HA1-His protein production either in the insect cell culture or larvae; in the study, the expressed protein was found to be glycosylated and may partly assemble into the trimeric form as its native structure, and it served as an effective subunit vaccine for immunization.
Declaration of conflicting interests
The authors declare no conflict of interest.
Acknowledgments
Acknowledgment
We are grateful to Prof. Ching-Ho Wang for the constructs and virus samples, and helpful suggestions. We also thank Dr. Yue-Long Wu and Cheng-Kang Tang for supplying the larvae, and Prof. Shih-Chung Chang for the pFastBac1 baculovirus expression vector.
Funding source
This work was supported by the Ministry of Science and Technology, Taiwan, ROC [grant numbers: 103-2321-B-002-060 and 104-2321-B-002-018].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2018.06.001.
Appendix A. Supplementary data
Supplementary material 1
References
- Chen Y.J., Chen W.S., Wu T.Y. Development of a bi-cistronic baculovirus expression vector by the Rhopalosiphum padi virus 5′ internal ribosome entry site. Biochem. Biophys. Res. Commun. 2005;335:616–623. doi: 10.1016/j.bbrc.2005.07.116. [DOI] [PubMed] [Google Scholar]
- Choi J.Y., Roh J.Y., Wang Y., Zhen Z., Tao X.Y., Lee J.H., Liu Q., Kim J.S., Shin S.W., Je Y.H. Analysis of genes expression of Spodoptera exigua larvae upon AcMNPV infection. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S., Wu C., Zhou H., Zhao R., Guo L., Wang J., Hung T. Secretory expression of all 16 subtypes of the hemagglutinin 1 protein of influenza A virus in insect cells. J. Virol. Methods. 2011;177:160–167. doi: 10.1016/j.jviromet.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Du L., Yang Y., Zhou Y., Lu L., Li F., Jiang S. MERS-CoV spike protein: a key target for antivirals. Expert Opin. Ther. Targets. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein Y., Faktor O., Elroy-Stein O., Levi B.Z. The use of bi-cistronic transfer vectors for the baculovirus expression system. J. Biotechnol. 1999;75:33–44. doi: 10.1016/s0168-1656(99)00131-5. [DOI] [PubMed] [Google Scholar]
- Franceschini F., Bottau P., Caimmi S., Crisafulli G., Lucia L., Peroni D., Saretta F., Vernich M., Povesi Dascola C., Caffarelli C. Vaccination in children with allergy to non active vaccine components. Clin. Transl. Med. 2015;4:3. doi: 10.1186/s40169-014-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin S.J., Skehel J.J. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J. Biol. Chem. 2010;285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.L., Jarvis D.L. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce "mammalianized" recombinant glycoproteins. Adv. Virus Res. 2006;68:159–191. doi: 10.1016/S0065-3527(06)68005-6. [DOI] [PubMed] [Google Scholar]
- He J.L., Hsieh M.S., Juang R.H., Wang C.H. A monoclonal antibody recognizes a highly conserved neutralizing epitope on hemagglutinin of H6N1 avian influenza virus. Vet. Microbiol. 2014;174:333–341. doi: 10.1016/j.vetmic.2014.10.008. [DOI] [PubMed] [Google Scholar]
- He J.L., Chiu Y.C., Chang S.C., Wang C.H., Juang R.H. Glycosylation at hemagglutinin Asn-167 protects the H6N1 avian influenza virus from tryptic cleavage at Arg-201 and maintains the viral infectivity. Virus Res. 2015;197:101–107. doi: 10.1016/j.virusres.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Hu Y.C., Luo Y.L., Ji W.T., Chulu J.L., Chang P.C., Shieh H., Wang C.Y., Liu H.J. Dual expression of the HA protein of H5N2 avian influenza virus in a baculovirus system. J. Virol. Methods. 2006;135:43–48. doi: 10.1016/j.jviromet.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kadkhodaei S., Rajabi Memari H., Abbasiliasi S., Akhavan Rezai M., Movahedi A., Joo Shun T., Bin Ariff A. Multiple overlap extension PCR (MOE-PCR): an effective technical shortcut to high throughput synthetic biology. RSC Adv. 2016;6:66682–66694. [Google Scholar]
- Kajikawa M. Silkworm Baculovirus expression system for molecular medicine. J. Biotechnol. Biomater. 2012;S9 [Google Scholar]
- Kato T., Kajikawa M., Maenaka K., Park E.Y. Silkworm expression system as a platform technology in life science. Appl. Microbiol. Biotechnol. 2010;85:459–470. doi: 10.1007/s00253-009-2267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuma S., Horie S., Daimon T., Iwanaga M., Shimada T. In vivo and in vitro analyses of a Bombyx mori nucleopolyhedrovirus mutant lacking functional vfgf. Virology. 2006;355:62–70. doi: 10.1016/j.virol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Condreay J.P., Jarvis D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Kawai T., Obinata M., Fujiwara H., Horiuchi T., Saeki Y., Sato Y., Furusawa M. Production of human alpha-interferon in silkworm using a baculovirus vector. Nature. 1985;315:592–594. doi: 10.1038/315592a0. [DOI] [PubMed] [Google Scholar]
- Mehle A. Unusual influenza a viruses in bats. Viruses. 2014;6:3438–3449. doi: 10.3390/v6093438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musthaq S.K., Kumar S.R., Szyporta M., Kwang J. Immunization with baculovirus displayed H6 hemagglutinin vaccine protects mice against lethal H6 influenza virus challenge. Antivir. Res. 2014;109:42–53. doi: 10.1016/j.antiviral.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Nunez L.F., Parra S.H., Mettifogo E., Catroxo M.H., Astolfi-Ferreira C.S., Piantino Ferreira A.J. Isolation of chicken astrovirus from specific pathogen-free chicken embryonated eggs. Poult. Sci. 2015;94:947–954. doi: 10.3382/ps/pev086. [DOI] [PubMed] [Google Scholar]
- Palache A., Oriol-Mathieu V., Fino M., Xydia-Charmanta M., Influenza Vaccine Supply Task Force Seasonal influenza vaccine dose distribution in 195 countries (2004-2013): little progress in estimated global vaccination coverage. Vaccine. 2015;33:5598–5605. doi: 10.1016/j.vaccine.2015.08.082. [DOI] [PubMed] [Google Scholar]
- Possee R.D., Howard S.C. Analysis of the polyhedrin gene promoter of the Autographa californica nuclear polyhedrosis virus. Nucleic Acids Res. 1987;15:10233–10248. doi: 10.1093/nar/15.24.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivkin H., Kroemer J.A., Bronshtein A., Belausov E., Webb B.A., Chejanovsky N. Response of immunocompetent and immunosuppressed Spodoptera littoralis larvae to baculovirus infection. J. Gen. Virol. 2006;87:2217–2225. doi: 10.1099/vir.0.81918-0. [DOI] [PubMed] [Google Scholar]
- Seok J.H., Kim J., Lee D.B., Cho K.J., Lee J.H., Bae G., Chung M.S., Kim K.H. Conformational modulation of influenza virus hemagglutinin: characterization and in vivo efficacy of monomeric form. Sci. Rep. 2017;7:7540–7549. doi: 10.1038/s41598-017-08021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Jarvis D.L. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets. 2007;8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza HA. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- Tuan S.J., Li N.J., Yeh C.C., Tang L.C., Chi H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 2014;107:897–905. doi: 10.1603/ec13435. [DOI] [PubMed] [Google Scholar]
- Van de Sandt C.E., Kreijtz J.H., Rimmelzwaan G.F. Evasion of influenza A viruses from innate and adaptive immune responses. Viruses. 2012;4:1438–1476. doi: 10.3390/v4091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.C., Chen J.R., Tseng Y.C., Hsu C.H., Hung Y.F., Chen S.W., Chen C.M., Khoo K.H., Cheng T.J., Cheng Y.S., Jan J.T., Wu C.Y., Ma C., Wong C.H. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18137–18142. doi: 10.1073/pnas.0909696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford M., Stewart S., Kuzio J., Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J. Virol. 1989;63:1393–1399. doi: 10.1128/jvi.63.3.1393-1399.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.Y., Chen Y.J., Teng C.Y., Chen W.S., Villaflores O. A bi-cistronic baculovirus expression vector for improved recombinant protein production. Bioeng. Bugs. 2012;3:129–132. doi: 10.4161/bbug.19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.G., Chung Y.C., Lai Y.K., Lai C.W., Liu H.J., Hu Y.C. Avian influenza virus hemagglutinin display on baculovirus envelope: cytoplasmic domain affects virus properties and vaccine potential. Mol. Ther. 2007;15:989–996. doi: 10.1038/mt.sj.6300131. [DOI] [PubMed] [Google Scholar]
- Zhou M.Y., Clark S.E., Gomez-Sanchez C.E. Universal cloning method by TA strategy. BioTechniques. 1995;19:34–35. [PubMed] [Google Scholar]
- Zhu X., Yu W., McBride R., Li Y., Chen L.M., Donis R.O., Tong S., Paulson J.C., Wilson I.A. Hemagglutinin homologue from H17N10 bat influenza virus exhibits divergent receptor-binding and pH-dependent fusion activities. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1458–1463. doi: 10.1073/pnas.1218509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1