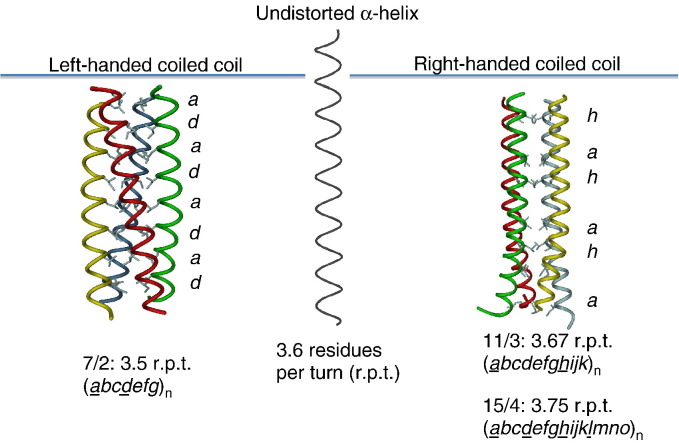
Fig. 1.
From α-helices to left- and right-handed coiled coils. In an undistorted α-helix one amino acid residue is rotated 100° along the screw axis and subsequently 3.6 residues are required for a full turn (centre). Coiled coils, which are pairs of α-helices winding around each other, show deviations from this rule. In left-handed coiled coils 7 residues are required for 2 turns and therefore are more tightly wound around each other in a left-handed supercoil to compensate for this missing 20° (left). In contrast, naturally occurring right-handed coiled coils reveal repeats of 11residues per 3 turns or 15 residues per 4 turns of the helix (right). As a consequence they are supercoiled either 20° or 60° to form a right-handed supercoil. Characteristic in all cases are highly repetitive sequence motifs of 7, 11, or 15 amino acid residues (in italics) with conserved aliphatic core residues (underlined) forming the hydrophobic core of coiled-coil channels.