Fig. 3.
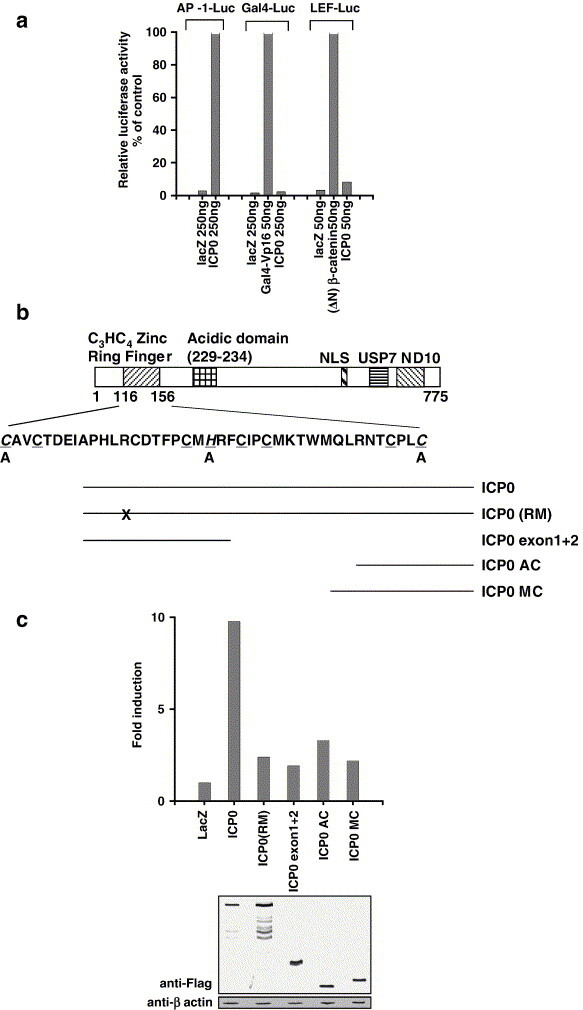
The RING domain of ICP0 is important for its selective activation of AP-1. (a) ICP0 activates AP-1-Luc, but not LEF-Luc or Gal4-Luc reporter genes. ICP0 or control plasmids were co-transfected into 293T cells along with the indicated reporter genes. (ΔN)-β-catenin and Gal4-VP16 are the cognate activators of LEF-Luc and Gal4-Luc, respectively. (b) Schematic diagram of ICP0 is indicated at the top. Positions of the C3HC4 Zinc Ring finger domain and the other domains were described. NLS—nuclear localization signal; USP7—sequences required for binding to the ubiquitin-specific protease USP7; ND10—sequences required for efficient localization at ND10 domains. Amino acid sequence of the C3HC4 Zinc Ring finger domain (aa 116–156) was shown. The consensus Zinc Ring finger sequence is underlined, and the mutations in this domain are in italics (aa 116 was changed from C to A, aa 136 from H to A and aa 156 from C to A). Diagrams of the ICP0 mutants are shown at the bottom. Black lines indicate the sequences retained in each mutant construct. ICP0 (RM): the Zinc Ring finger domain is site-mutated, ‘X’ indicates the mutations; ICP0 exon1 + 2: N-terminus of ICP0 harboring the RING domain; ICP0 AC and ICP0 MC: C-terminal parts of ICP0 that do not contain the RING domain (see details of these plasmids in Materials and methods). (c) AP-1-Luc activation induced by truncations of ICP0. Flag-tagged-variants of ICP0 were co-transfected into 293T cells along with the AP-1 reporter gene and internal control respectively. 48 h later, cells were collected, and dual-luciferase assay was performed.
