Abstract
Numerous adjunct therapeutic agents have been investigated for the treatment or control of fat mobilization syndrome in periparturient dairy cows. The aim of this study was to determine the effects of multiple i.v. injections of 10% butaphosphan and 0.005% cyanocobalamin combination (Catosal, Bayer Animal Health, Leverkusen, Germany) between 1 and 2 wk antepartum (a.p.) on the metabolism and health of dairy cows. Forty-five late-gestation Holstein-Friesian cows (second pregnancy) were allocated randomly to 1 of 3 groups with 15 cows/group: group C6 (6 daily i.v. injections of butaphosphan at 10 mg/kg of body weight (BW) and cyanocobalamin at 5 μg/kg of BW in the last 2 wk of gestation); group C3 (3 daily i.v. injections of butaphosphan at 10 mg/kg of BW and cyanocobalamin at 5 μg/kg of BW in the last week of gestation); and group C0 (equivolume daily i.v. injections of 0.9% NaCl solution). Serum biochemical analysis was performed on jugular venous blood samples that were periodically obtained a.p. and postpartum (p.p.). Health status and milk production were monitored p.p. Serum cyanocobalamin concentration increased in groups C6 and C3 p.p. Multiple daily i.v. injections of Catosal before parturition increased p.p. glucose availability, as evaluated by p.p. serum glucose concentration, and decreased peripheral fat mobilization and ketone body formation, as evaluated by p.p. serum nonesterified fatty acid and β-OH butyrate concentrations. The number of puerperal infections in the first 5 d after calving was decreased in group C6, relative to group C0. We conclude that multiple injections of Catosal during the close-up period have a beneficial effect on the metabolism of periparturient dairy cows. Our results are consistent with the hypothesis that high-producing dairy cows in early lactation may have a relative or actual deficiency of cyanocobalamin.
Key words: ketosis, hepatic lipidosis, butaphosphan, cyanocobalamin
Introduction
A transient and acute state of negative energy balance and inadequate DMI in the periparturient period can lead to mobilization of large amounts of body fat, a concomitant increase in plasma NEFA concentration, and accumulation of lipids in the liver of dairy cows (Fürll, 1989; Grummer, 2008). Enhanced lipolysis in the periparturient period was first identified in the 1970s and was termed fat mobilization syndrome by Morrow in 1976 (Morrow, 1976). Increased lipolysis in the periparturient dairy cow is accompanied by an increased incidence of clinically important diseases such as ketosis, retained placenta, metritis, mastitis, abomasal displacement, and reduced immune resistance (Morrow, 1976; Curtis et al., 1985; Gerloff et al., 1986). Fat mobilization syndrome is characterized by periparturient increases in serum NEFA and BHBA concentrations, and decreases in serum glucose, insulin, and IGF-1 concentrations (Aeberhard et al., 2001; Fürll and Jäckel, 2006). The syndrome is associated with decreased productivity and reproductive performance (Butler, 2000; Leroy et al., 2006) and increased morbidity and death in severely affected animals.
The treatment and prevention of fat mobilization syndrome has focused on maximizing energy and DMI in the periparturient period. Numerous adjunct therapeutic agents that decrease periparturient lipolysis, promote fatty acid oxidation in hepatic mitochondria, or facilitate the export of lipoproteins from the liver have been investigated as adjunct agents for the treatment or control of fat mobilization syndrome. Studies in periparturient dairy cows have evaluated the effect of orally administered propylene glycol and propionate (Kristensen and Raun, 2007; Grummer, 2008), glycerol (DeFrain et al., 2004), nicotinic acid (Fürll, 1989; Drackley et al., 1998; Pires et al., 2007), cis-linoleic acid (Selberg et al., 2004; Mosley et al., 2007), fat (Drackley et al., 1998; Moallem et al., 2007), methionine (Preynat et al., 2009), choline (Chung et al., 2009), carnitine (LaCount et al., 1995; Carlson et al., 2007), and monensin (Arieli et al., 2008; Duffield et al., 2008) on fat mobilization syndrome. Other potential adjunctive treatments, such as insulin, glucagon, and dexamethasone, have been evaluated but suffer from several associated problems that affect their current use (Fürll and Jäckel, 2005; Nafikov et al., 2006). There is a continuing need for an adjunct therapeutic agent that is effective in the treatment or prevention of fat mobilization syndrome in periparturient dairy cows.
Catosal (Bayer Animal Health, Leverkusen, Germany) is a commercially available mixture of cyanocobalamin (50 μg of cyanocobalamin/mL) and a phosphorus-containing compound (100 mg of butaphosphan/mL) that is labeled for the improvement of energy metabolism in cattle and pigs in 60 countries and as a source of cyanocobalamin and phosphorus for prevention or treatment of deficiencies of these nutrients in cattle, swine, horses, and poultry in the United States. The label dose of Catosal in the United States is 1 to 2 mL/100 lb of BW by i.v., i.m., or s.c. injection, equivalent to butaphosphan (0.45–0.91 mg/kg of BW) and cyanocobalamin (0.23–0.45 μg/kg of BW), and the injection can be repeated daily. Increased milk and milk protein yields have been observed following intramuscular cyanocobalamin administration (10 mg/cow per week) and oral cyanocobalamin supplementation (500 mg/cow per day; Girard and Matte, 2005; Graulet et al., 2007), but the effects on metabolism have been variable (Schuh, 1994; Fürll et al., 2006; Lohr et al., 2006). In dairy cattle, the phosphorus content of liver tissue decreases in early lactation due to a reduction in hepatocellular cytosol volume as well as a decrease in cytosolic phosphorus concentration (Grünberg et al., 2009). Phosphorus supplementation may therefore be indicated in periparturient dairy cows because phosphorus plays an important role in hepatic carbohydrate metabolism, in which all intermediates in the gluconeogenic pathway must be phosphorylated, and because the rates of gluconeogenesis and glycolysis are regulated by phosphorus availability (Berg et al., 2006). Several reports indicate a reduction in morbidity following administration of butaphosphan and cyanocobalamin (Sommer et al., 1971; Flasshoff, 1974; Palmer, 1980; Schuh, 1994), and a recent report indicated that injection of butaphosphan and cyanocobalamin on the day of calving and 1 d later decreased the prevalence of subclinical ketosis during the week after calving in mature dairy cows (Rollin et al., 2010). We therefore hypothesized that parenteral administration of Catosal in the late dry period would improve energy metabolism in periparturient dairy cattle. Accordingly, the main aim of this study was to determine the effects of multiple i.v. injections of a cyanocobalamin and butaphosphan combination (Catosal) administered between 1 and 2 wk antepartum (a.p.) on the metabolism of dairy cows.
Materials and Methods
Animals
The study was conducted in a Holstein-Friesian herd under standardized housing and environmental conditions. The herd of 1,500 dairy cows had a 365-d milk yield of 10,100 L/cow. Cows were kept in loose housing with slatted floors and separated into 4 pens of similar milk yield (dry period, close up, 0–50 d lactation, 51 and following days). Cows were milked twice daily using a side-by-side 2 × 24 system (Xpressway supreme, BouMatic Gascoigne Melotte, Remicourt, Belgium), a cow identification system (Cow Trakker, BouMatic Gascoigne Melotte), and automatic recording of milk volumes (Perfection 3000, BouMatic Gascoigne Melotte).
Forty-five cows in late gestation (second pregnancy) were admitted to the study on a randomized basis (Schulz and Grimes, 2002). The cows were dried off 6 wk a.p. and were clinically healthy. The feeding routine and composition were standardized (Table 1 ). All cows were administered a standard immunization program in late gestation to protect their calves against Escherichia coli, Salmonella typhimurium, rotavirus, and coronavirus infection. Clinically ill cows were excluded from the study.
Table 1.
Ingredients and nutrient composition of the diets fed to dairy cows
| Item | Dry cows | Close-up cows | Lactation cows |
|---|---|---|---|
| Ingredient (%) | |||
| Grass hay | 88.01 | 26.21 | 7.02 |
| Legume-grass silage3 | — | 24.2 | 27.3 |
| Corn silage4 | — | 18.0 | 18.2 |
| Cracked corn | 7.8 | 22.9 | 32.4 |
| Soybean meal, 49% | 2.5 | 5.5 | 8.6 |
| Micronized soybean | — | 0.31 | 0.74 |
| Distillers grain (wheat) | — | 0.32 | 0.77 |
| Distillers grain (corn) | — | 0.24 | 0.57 |
| Corn gluten meal | — | 0.40 | 0.95 |
| Canola meal | — | 0.32 | 0.77 |
| Mineral and vitamin premix | 1.35 | 1.55 | 2.36 |
| Calcium carbonate | 0.4 | — | 0.4 |
| Nutrient composition | |||
| DM (%) | 85.5 | 46.8 | 44.4 |
| CP (%) | 9.4 | 13.8 | 16.4 |
| RDP7 (%) | 5.9 | 9.7 | 11.0 |
| RUP7 (%) | 3.6 | 4.2 | 5.4 |
| ADF (%) | 36.4 | 24.3 | 18.5 |
| NDF (%) | 61.8 | 40.8 | 31.1 |
| NEL8 (Mcal/d) | 14.6 | 18.2 | 33.9 |
| MP8 (g/d) | 664 | 1,019 | 2,211 |
| Met8 (% of MP) | 1.86 | 1.97 | 1.87 |
| Lys8 (% of MP) | 6.53 | 6.86 | 6.44 |
| K (%) | 2.07 | 1.75 | 1.38 |
| Ca (%) | 0.71 | 0.62 | 0.84 |
| P (%) | 0.46 | 0.48 | 0.48 |
| Mg (%) | 0.32 | 0.38 | 0.30 |
| S (%) | 0.24 | 0.23 | 0.23 |
| Cl (%) | 0.53 | 0.45 | 0.47 |
| Co (mg/kg) | 1.71 | 1.89 | 0.84 |
8.3% CP, 1.0% ADF – CP, 3.0% NDF – CP, 3.4% soluble protein, 40.9% ADF, 69.1% NDF, 4.9% lignin.
13.4% CP, 1.0% ADF – CP, 4.0% NDF – CP, 5.7% soluble protein, 40.9% ADF, 69.1% NDF, 4.9% lignin.
18.5% CP, 1.6% ADF – CP, 4.8% NDF – CP, 8.4% soluble protein, 34.4% ADF, 54.0% NDF, 3.0% lignin.
8.8% CP, 0.6% ADF – CP, 1.2% NDF – CP, 5.1% soluble protein, 21.3% ADF, 35.1% NDF, 1.9% lignin.
Contains the following per kilogram: 31.3 g of Ca, 114 g of P, 120 g of Mg, 24 g of Na, 105 g of Cl, 16 g of K, 20 g of S, 4,453 mg of Fe, 6,500 mg of Mn, 7,700 mg of Zn, 1,610 mg of Cu, 202 mg of I, 120 mg of Co, 40 mg of Se (25% organic Se), 800,000 IU of vitamin A, 245,000 IU of vitamin D, and 7,500 IU of vitamin E.
Contains the following per kilogram: 67.8 g of Ca, 66.4 g of P, 39 g of Mg, 151 g of Na, 108 g of Cl, 6.7 g of K, 10 g of S, 2,887 mg of Fe, 2,004 mg of Mn, 1,896 mg of Zn, 462.6 mg of Cu, 57.6 mg of I, 34.2 mg of Co, 24 mg of Se (25% organic Se), 398,800 IU of vitamin A, 70,458 IU of vitamin D, and 2,293 IU of vitamin E.
Calculated according to the NRC model (NRC, 2001).
Calculated according to the NRC model (NRC, 2001) from averaged DMI of control cows (B9–B12–) during the drying (11.0 kg/d), close-up (11.4 kg/d), and lactation (21.3 kg/d) periods.
Experimental Protocol
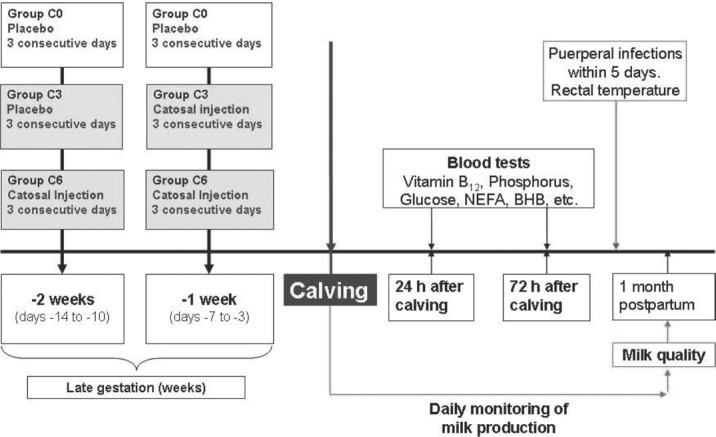
Cows were randomly assigned to 1 of 3 groups using the random number generation data analysis tool in Microsoft Office Excel 2003 SP3 (www.microsoft.com). The treatment and sampling plan is shown in Figure 1 . The first group (C6, n = 15) received a total of 6 Catosal (10% butaphosphan and 0.005% cyanocobalamin combination, Bayer Animal Health) i.v. injections (10 mL/100 kg of BW); these were administered as 3 sequential i.v. injections every 24 h from 14 to 10 d a.p. and from 7 to 3 d a.p. (Figure 1). The second group (C3, n = 15) received 3 sequential 0.9% NaCl (10 mL/100 kg of BW) i.v. injections every 24 h from 14 to 10 d a.p. and 3 sequential Catosal i.v. injections every 24 h from 7 to 3 d a.p. The third group (C0, n = 15) were administered 6 i.v. injections of 0.9% NaCl (10 mL/100 kg BW) every 24 h from 14 to 10 d a.p. and from 7 to 3 d a.p. The daily dosage of Catosal to cows in groups C6 and C3 (butaphosphan, 10 mg/kg of BW; cyanocobalamin, 5 μg/kg of BW) was approximately 11 times higher than the upper range of the labeled dose for daily administration of butaphosphan (0.91 mg/kg of BW) and cyanocobalamin (0.45 μg/kg of BW) to cattle in the United States.
Figure 1.
Experimental protocol: treatment and sampling plan.
Blood was collected from the external jugular vein into plain glass vacutainer tubes in the morning immediately before the first and second treatments with Catosal or placebo and on d 1 and 3 p.p. The p.p. time points were selected because they spanned the time interval of greatest metabolic stress after parturition (Morrow, 1976; Gerloff et al., 1986; Fürll, 1989). This provided a total of 4 time points for serum biochemical analysis.
Cows were monitored clinically up to d 20 of lactation p.p. Rectal temperatures were measured twice daily for the first 5 d p.p., and the presence of puerperal infection was defined by a rectal body temperature ≥39.6°C. Milk volume was recorded daily for the first month p.p. and milk composition (fat, protein, lactose) was determined at one time during the first month p.p. as part of the government milk yield monitoring program. Milk fat, protein, and lactose concentrations were measured using infrared spectroscopy (Milkoscan FT+, Foss Analytical, Hillerød, Denmark). Milk SCC was measured using an optical fluorescent method (Fossomatic FC 500, Foss Analytical).
Blood Samples
Jugular venous blood was allowed to clot at room temperature, centrifuged at 3,800 × g, and the serum harvested and stored at −18 to −20°C; serum was thawed at room temperature immediately before analysis. The following metabolic parameters were determined using an automatic analyzer (Hitachi 912 automated chemistry analyzer, Boehringer Mannheim, Mannheim, Germany): NEFA (colorimetric method based on the reaction of ATP and coenzyme A with NEFA), BHBA (measured following conversion to acetoacetate, which in turn converts NAD to NADH), glucose (glucose hexokinase), total bilirubin (diazotized sulfanilic acid), and cholesterol (colorimetric) concentrations, as well as the serum activities of aspartate aminotransferase (AST; aspartate Tris buffer method), alkaline phosphatase (ALP, p-nitrophenol method), and creatine kinase (CK, N-acetyl-l-cysteine–activated UV method). The following serum concentrations were also determined: cyanocobalamin (electrochemiluminescence immunoassay, Roche/Hitachi Modular E170, Basel, Switzerland), insulin (INS-immunoradiometric RIA, Biosource Europe S.A., Hamburg, Germany), cortisol (RIA, Abraham et al., 1972), cobalt, and selenium (flow injection analysis system–transversely heated graphite atomizer coupling, graphite tube-AAS 4100ZL, Perkin Elmer, Rodgau-Jügesheim, Germany).
Statistical Evaluation
Data were expressed as mean ± standard deviation or as geometric mean and 95% CI; P < 0.05 was considered significant. Repeated-measures ANOVA using PROC MIXED (SAS 9.2, SAS Institute Inc., Cary, NC) and an autoregressive covariance structure was used to determine the main effects of group (3 levels), time (4 levels), and the interaction between group and time. Values for serum cyanocobalamin, phosphorus, glucose, NEFA, total bilirubin, cortisol and insulin concentrations, and serum CK and AST activities were log-transformed to achieve homogeneous variances and meet one of the assumptions of ANOVA. Bonferroni-adjusted P-values were used to assess differences between groups C6 and C3 and C0 at each time point whenever the F-test for group or group × time interaction was significant. Bonferroni-adjusted P-values were used to assess changes over time to the day = −14 d value for each group whenever the F-test for time or group × time interaction was significant. Fisher's exact test (PROC FREQ; SAS 9.2, SAS Institute) was used to compare the number of cows in each group with puerperal infections. Analysis of variance using PROC GLM (SAS 9.2, SAS Institute) with DIM at sampling as a covariate was used to determine the main effects of group (3 levels) on milk production, log10 SCC, fat percentage, protein percentage, and lactose concentration at the first herd test after parturition.
Results
Serum Biochemical Analysis
Significant group effects were present for serum cyanocobalamin (P < 0.0001), total bilirubin (P = 0.013), glucose (P = 0.039), and BHBA (P = 0.049) concentrations. As expected for periparturient dairy cattle, there was a significant time effect for all serum biochemical variables measured. Significant group × time interactions were present for serum cyanocobalamin (P < 0.0001), total bilirubin (P = 0.0005), NEFA (P = 0.0019), and glucose (P = 0.027) concentrations.
The serum concentration of cyanocobalamin was increased in groups C6 and C3 compared with cows in the untreated group (C0) (Figure 2 ). There were no differences in serum cobalt concentration in all 3 groups on d 14 a.p. and d 3 p.p. (data not shown). The serum phosphorus concentration was decreased on d 1 p.p and d 3 p.p. in groups C3 and C0, but not in group C6.
Figure 2.
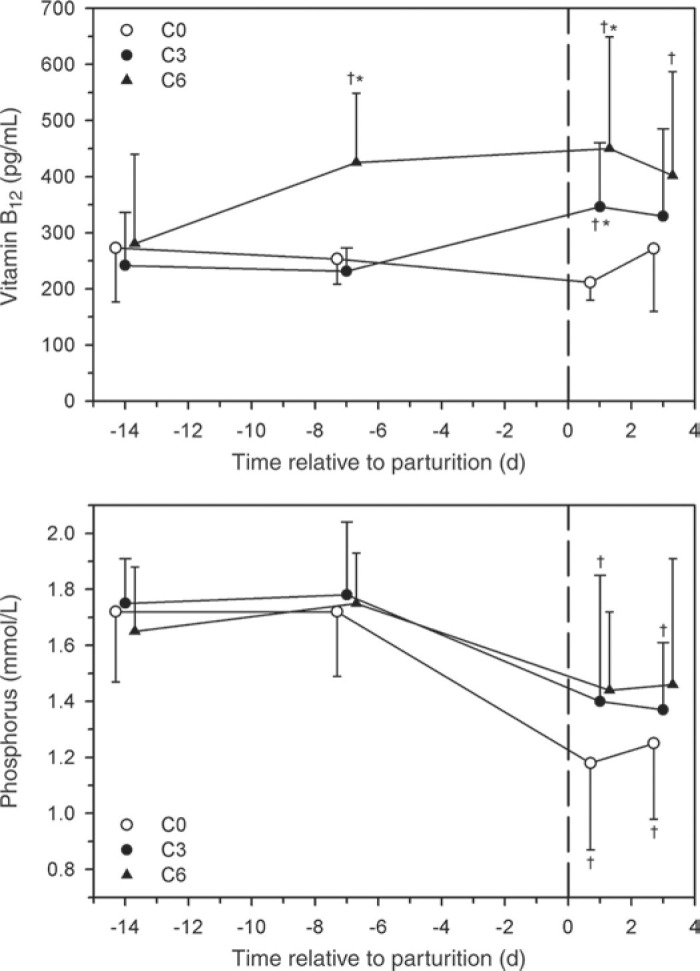
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on the serum cyanocobalamin and phosphorus concentrations of dairy cows. Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD; *P < 0.25 (Bonferroni adjusted) from group C0 at the same time; †P < 0.0167 (Bonferroni adjusted) from time = −14 d value.
The serum glucose concentration was higher in group C6 than in group C0 on d 1 p.p. (Figure 3 ). The serum NEFA concentrations were increased after calving in all 3 groups, but were lower in group C6 at d 1 and 3 p.p. than in group C0. The serum BHBA concentrations were increased after calving in groups C3 and C0, but not group C6, and were lower in group C6 at d 1 p.p. than in group C0.
Figure 3.
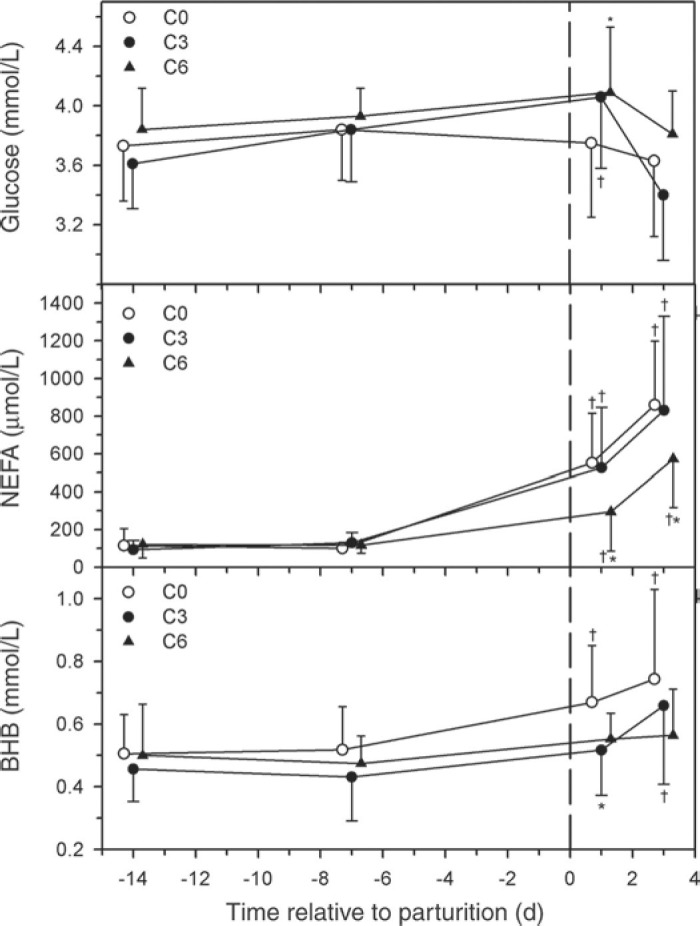
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on serum glucose, NEFA, and BHBA concentrations of dairy cows. Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD *P < 0.25 (Bonferroni adjusted) from group C0 at the same time. †P < 0.025 (Bonferroni adjusted) from time = −14 d value.
The serum cortisol concentration was increased after calving with no differences between groups (Figure 4 ). The serum insulin concentration was decreased after calving in all 3 groups. The serum total bilirubin concentrations increased after calving in all 3 groups; however, serum total bilirubin concentration was lower in group C6 at d 1 and 3 p.p. than in group C0 (Figure 5 ). The serum calcium concentrations were decreased in all 3 groups on d 1 p.p. with no differences between groups. No significant differences in serum cholesterol, magnesium, and selenium concentrations or serum AST, ALP, and CK activities between groups were observed at the 4 time points (data not shown).
Figure 4.
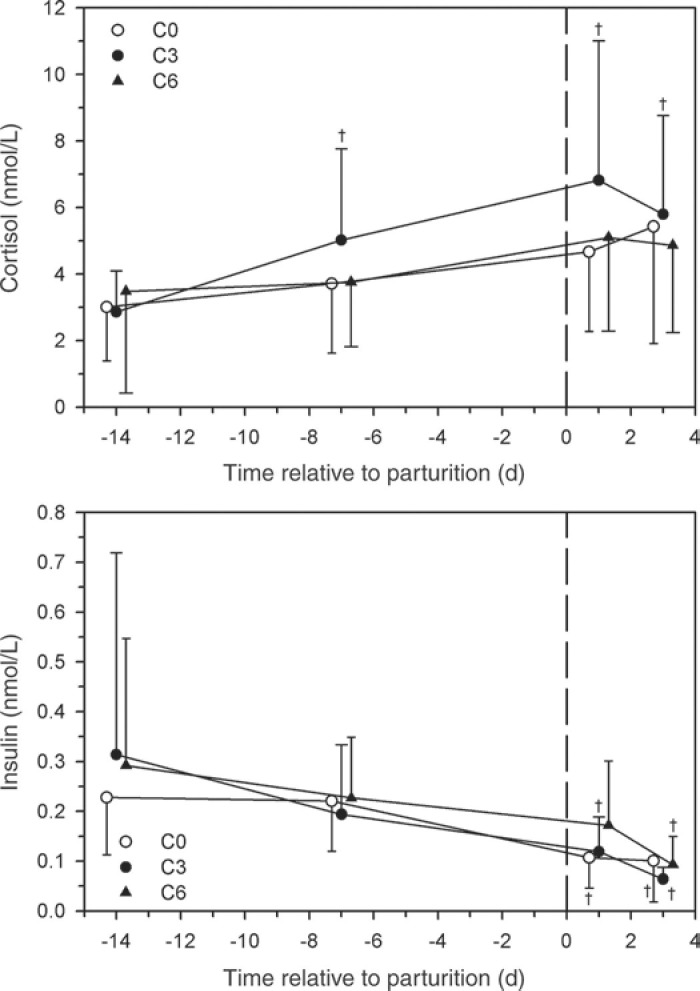
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on the serum cortisol and insulin concentrations of dairy cows. Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD †P < 0.025 (Bonferroni adjusted) from time = −14 d value.
Figure 5.
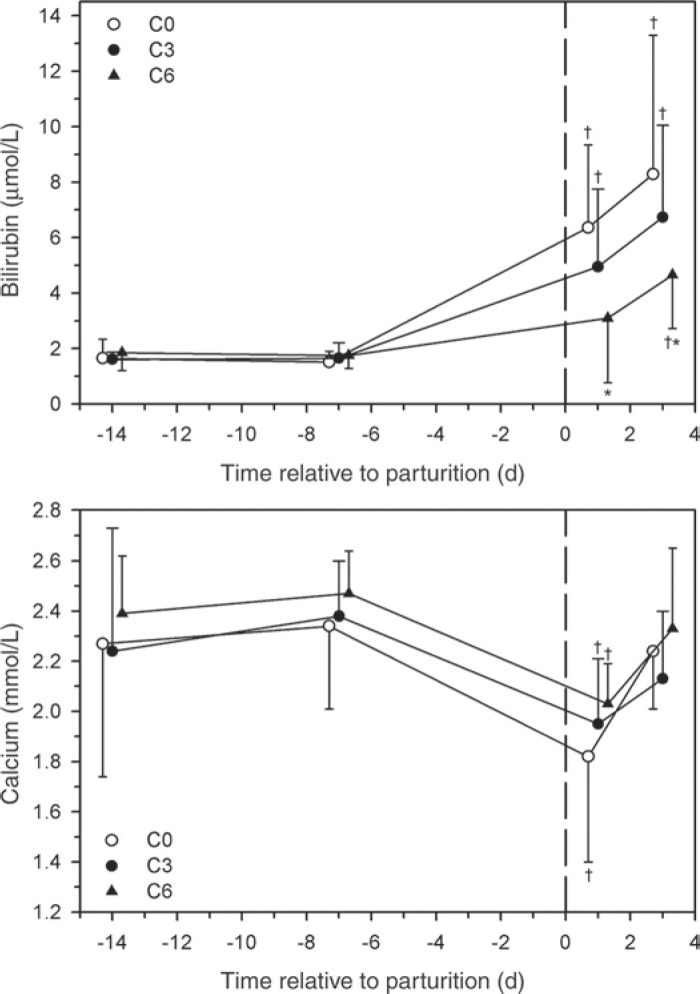
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on the serum total bilirubin and calcium concentrations of dairy cows. Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD *P < 0.025 (Bonferroni adjusted) from group C0 at the same time. †P < 0.0167 (Bonferroni adjusted) from time = −14 d value.
Clinical Findings
Puerperal infections in the first 5 d were present in 2 cows in the C6 group (13%; P < 0.025 compared with control group), in 3 cows in the C3 group (24%; P = 0.079 compared with control group), and in 8 cows in the control group (53%). Cows in the C6 group therefore received the fewest intrauterine or injectable antimicrobial treatments.
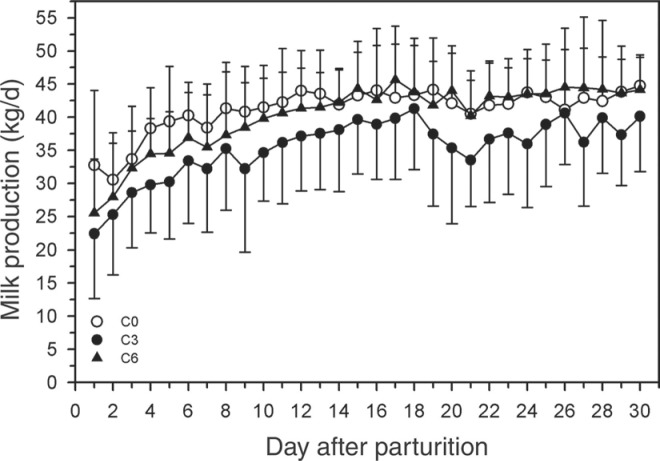
There were no differences in daily or cumulative milk yield between the 3 groups (Figure 6 ). Cows in the C6 group had the highest lactose concentration in the first milk test after calving adjusted for days in milk (Table 2 ).
Figure 6.
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on daily milk production of dairy cows for the first month of lactation. Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD.
Table 2.
Effect of multiple i.v. injections of cyanocobalamin and butaphosphan administered between 1 and 2 wk antepartum (a.p.) on milk production, SCC, fat and protein percentage, and lactose concentration of lactating dairy cows at the first herd test after parturition1
| Group | DIM at sampling | Milk production (kg/d) | SCC (cells/mL) | Milk fat (%) | Milk protein (%) | Milk lactose (g/L) |
|---|---|---|---|---|---|---|
| C6 | 20.6 ± 7.9 | 44.0 ± 7.3 | 78,162 (2,420 to 2,524,643) |
4.13 ± 0.56 | 3.30 ± 0.26 | 49.2 ± 1.57* |
| C3 | 22.8 ± 12.0 | 37.3 ± 9.0 | 47,206 (9,132 to 244,028) |
4.89 ± 1.15 | 3.41 ± 0.48 | 47.4 ± 2.42 |
| C0 | 19.1 ± 8.4 | 42.9 ± 6.3 | 57,544 (3,768 to 878,699) |
4.68 ± 1.00 | 3.19 ± 0.32 | 47.6 ± 1.97 |
Forty-five late-gestation Holstein-Friesian cows were allocated randomly to 1 of 3 groups (15 cows/group): group C6 (6 i.v. injections of cyanocobalamin and butaphosphan in the last 2 wk of gestation); group C3 (3 i.v. injections of cyanocobalamin and butaphosphan in the last week of gestation); group C0 (equivolume i.v. injections of 0.9% NaCl solution). Data are mean ± SD except SCC, which is geometric mean (95% CI in parentheses).
P < 0.025 (Bonferroni adjusted) from group C0.
Discussion
The major findings of the study reported here were that multiple i.v. injections of cyanocobalamin and butaphosphan before parturition increased glucose availability, as evaluated by p.p. serum glucose concentration, decreased peripheral fat mobilization and ketone body formation, as evaluated by p.p. serum NEFA and BHBA concentrations at the time of greatest metabolic stress (Morrow, 1976; Gerloff et al., 1986; Fürll, 1989), and decreased the number of puerperal infections. The improvement in p.p. metabolic status was temporally associated with increased serum cyanocobalamin concentrations a.p. Although an association does not prove a causal relationship, a causal relationship is likely when 2 events are chronologically linked, a dose-dependent relationship is identified, and there is a biologically plausible mechanism for the relationship.
Our results are consistent with the hypothesis that high-producing dairy cows in early lactation may have a relative or actual deficiency of cyanocobalamin (Girard and Matte, 2005) and suggest that cyanocobalamin deficiency may contribute to the development of fat mobilization syndrome. Serum cyanocobalamin concentrations are low in early lactation (Girard and Matte, 2005), and cyanocobalamin supply is considered a limiting factor for lactation performance. Methylmalonyl-CoA mutase is a cyanocobalamin-dependent enzyme that transforms methylmalonyl-CoA into succinyl-CoA; cyanocobalamin therefore plays a pivotal role in the entry of propionate into the Krebs cycle and the utilization of propionate for gluconeogenesis (Mykkänen and Korpela, 1981; Girard and Matte, 2005). These observations directly lead to the hypothesis that cyanocobalamin deficiency contributes to fat mobilization syndrome by decreasing the rate of propionate utilization for gluconeogenesis. The cyanocobalamin requirement of dairy cattle is 10 to 30 mg/d (Steinberg and Klünter, 1995). Most of this daily requirement is provided by the metabolic activity of ruminal bacteria, which produce a mean of 73 mg of cyanocobalamin per day in lactating Holstein-Friesian cows; however, the oral bioavailability of cyanocobalamin is low (Santschi et al., 2005). For comparison, cows in the C6 and C3 groups in the study reported here received 5 mg of cyanocobalamin i.v. daily for 6 or 3 d a.p., respectively, which increased serum cyanocobalamin concentration in a dose-dependent manner. As such, the dose of cyanocobalamin administered to the cows in the study reported here was biologically relevant.
Cyanocobalamin is essential for gluconeogenesis from propionate (Mykkänen and Korpela, 1981; Girard and Matte, 2005), and a relative deficiency of cyanocobalamin would be expected to decrease glucose availability and affect bioenergetics and lactose synthesis because propionate is the major gluconeogenic substrate in high-yielding dairy cows (Preynat et al., 2009). The higher serum glucose and insulin concentrations, the lower serum NEFA and BHBA concentrations, and higher milk lactose concentration in group C6 cows after calving in this study are consistent with increased glucose availability or increased DMI, although neither factor was directly measured in the study reported here. The results of 2 studies in lactating dairy cows indicate that cyanocobalamin administration increased glucose availability and lactational performance in the absence of an increase in DMI (Girard and Matte, 2005; Preynat et al., 2009).
Our results should be compared with those of 7 previous studies in dairy cows that utilized the same formulation of cyanocobalamin and butaphosphan (Sommer et al., 1971; Flasshoff, 1974; Palmer, 1980; Schuh, 1994; Fürll et al., 2006; Lohr et al., 2006; Rollin et al., 2010). The 7 previous studies administered s.c. or i.v doses of cyanocobalamin (1–4 mg/cow) and butaphosphan (2–8 g/cow) on 1 to 3 occasions; approximate i.v. doses of cyanocobalamin (3 mg/cow) and butaphosphan (6 g/cow) were administered on 6 (group C6) or 3 (group C3) occasions to cows in the study reported here. The results of previously published studies, and a comparison of the effects of the 2 different dosage protocols used in the study reported here, indicate that the effects of cyanocobalamin and butaphosphan combination are dose-dependent and that a bioenergetically favorable response may require at least 3 i.v. injections of at least 3 mg of cyanocobalamin and 6 g of butaphosphan for each cow. It should be noted that the dry period of cows in the study reported here was 42 d. It is unknown whether treatment of cows with a longer dry period, which are more typical on some dairies, would have led to a different result.
Butaphosphan is an α-amino phosphonic acid derivative that contains phosphorus. The precise mode of action of butaphosphan on metabolism is unknown, and the direct effect of butaphosphan on phosphorus homeostasis in animals remains unknown (EMEA, 1999). It is questionable whether the effect of butaphosphan is simply a matter of the substitution of phosphorus (EMEA, 1999), and butaphosphan injection to the cows in the study reported here did not immediately result in an increase in the serum phosphorus concentration (Figure 2). However, serum phosphorus and calcium concentrations were numerically higher in group C6 and C3 cows at p.p. d 1 than in group C0 cows (Figure 2; Figure 5); this result most likely reflects the numerically higher milk production on the first day after parturition in group C0 cows because lactation is a major drain on calcium and phosphorus. The numerically higher serum calcium and phosphorus concentrations in group C6 and C3 cows at p.p. d 1 may have also reflected improved overall health rather than a direct effect of butaphosphan on serum phosphorus concentration because serum phosphorus concentration is decreased in early lactating dairy cows with decreased feed intake and gastrointestinal motility (Gruenberg et al., 2005). Serum calcium and phosphorus concentrations on d 1 and the first week after calving were not affected by s.c. administration of 25 mL of Catosal on the day of calving and 1 d later (Rollin et al., 2010), supporting the concept that the elemental phosphorus in butaphosphan does not directly increase serum phosphorus concentrations in dairy cattle. The major label indications for butaphosphan are to treat disorders of metabolism and to support the treatment of infertility, tetany, and paresis as an adjunct to calcium and magnesium therapy (EMEA, 1999, 2000). Butaphosphan is rapidly eliminated primarily via the urinary tract after i.v. administration, with a terminal half-life of 116 min in lactating dairy cows (EMEA, 2000). Although butaphosphan administration did not alter basal serum insulin or cortisol concentration in the study reported here (Figure 4), butaphosphan has been shown to facilitate insulin release and glucose entry into insulin-dependent cells and decrease cortisol release in nonlactating dairy heifers (Hansel et al., 1992) and piglets undergoing social stress (van der Staay et al., 2007) through an unidentified mechanism. As such, butaphosphan may have contributed to the beneficial effects of Catosal administration to cows in groups C6 and C3. Additional investigations are necessary to clarify a possible synergistic action of butaphosphan and cyanocobalamin on energy metabolism.
We believe it is helpful to compare the effects of other adjunct treatments for the periparturient stabilization of metabolism (such as oral propylene glycol, propionate, glycerol, nicotinic acid, cis-linoleic acid, fat, methionine, choline, carnitine, monensin) to the decrease in NEFA concentrations at 1 and 3 d p.p. achieved in this study in group C6. Daily doses of propylene glycol exceeding 500 to 1,000 mL (or equivalent when dry propylene glycol formulations are fed) are required to significantly change serum NEFA, glucose, and insulin concentrations in periparturient dairy cattle (Kristensen and Raun, 2007; Grummer, 2008); doses of this magnitude are not currently approved within the European Union. The protective effects of glycerol supplements for transition cow feeding are variable (DeFrain et al., 2004), and the consensus is that propylene glycol provides a superior and more predictable metabolic response than glycerol in periparturient dairy cows. The metabolic effects of nicotinic acid are dose-dependent (Drackley et al., 1998; Pires et al., 2007). Although cis-linoleic acid supplementation can decrease milk fat content (Selberg et al., 2004; Mosley et al., 2007), the metabolic effects of this treatment are unclear. Adding fat to ruminant rations can be effective in stabilizing energy metabolism, although decreased DMI and increased serum NEFA and BHBA concentrations have been described (Moallem et al., 2007). Neither rumen-protected methionine (Preynat et al., 2009) or choline (Chung et al., 2009) altered glucose kinetics or plasma NEFA and BHBA concentrations in lactating dairy cows, although rumen-protected methionine decreased milk lactose concentration and increased milk protein concentration (Preynat et al., 2009). Carnitine supplementation and monensin administration do not result in marked decreases in serum NEFA and BHBA concentrations in periparturient cows (LaCount et al., 1995; Carlson et al., 2007; Arieli et al., 2008; Duffield et al., 2008). Dexamethasone, which is used to great effect postpartum in stabilizing metabolism and decreasing serum NEFA and bilirubin concentrations (Fürll and Jäckel, 2005), cannot be used a.p. because of its effect on inducing parturition and increasing the incidence of retained placenta.
In summary, multiple i.v. injections of cyanocobalamin and butaphosphan in a commercially available formulation (Catosal) during the close-up period have a beneficial effect on the metabolism of periparturient dairy cows. Our results are consistent with the hypothesis that high-producing dairy cows in early lactation may have a relative or actual deficiency of cyanocobalamin. Additional studies are indicated to investigate the potential synergistic effects of cyanocobalamin and butaphosphan in improving the metabolic status and health of dairy cows in early lactation, whether cyanocobalamin and butaphosphan increase DMI in periparturient dairy cows, and whether the beneficial effect of Catosal is present when multiple injections are administered a.p. at the labeled dose for cattle in the United States.
References
- Abraham G.E., Buster J.E., Teller R.C. Radioimmunoassay for plasma cortisol. Anal. Lett. 1972;5:757–758. [Google Scholar]
- Aeberhard K., Bruckmaier R.M., Kuepfer U., Blum J.W. Milk yield and composition, nutrition, body conformation traits, body condition scores, fertility and diseases in high-yielding dairy cows–Part 1. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001;48:97–110. doi: 10.1046/j.1439-0442.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- NRC . 7th ed. National Academy Press; Washington DC: 2001. Nutrient Requirements of Dairy Cattle. [Google Scholar]
- Arieli A., Dicken U., Dagoni I., Spirer Y., Zamwel S. Production and health of cows given monensin prepartum and a high-energy diet postpartum. J. Dairy Sci. 2008;91:1845–1851. doi: 10.3168/jds.2007-0795. [DOI] [PubMed] [Google Scholar]
- Berg J.M., Tymoczo J.L., Stryer L. Glycolysis and gluconeogenesis. In: Berg J.M., Tymoczo J.L., Stryer L., editors. Biochemistry. 6th ed. W. H. Freeman and Company; New York, NY: 2006. pp. 433–474. [Google Scholar]
- Butler W.R. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000;60–61:449–457. doi: 10.1016/s0378-4320(00)00076-2. (Review) [DOI] [PubMed] [Google Scholar]
- Carlson D.B., McFadden J.W., D’Angelo A., Woodworth J.C., Drackley J.K. Dietary L-carnitine affects periparturient nutrient metabolism and lactation in multiparous cows. J. Dairy Sci. 2007;90:3422–3441. doi: 10.3168/jds.2006-811. [DOI] [PubMed] [Google Scholar]
- Chung Y.H., Brown N.E., Martinez C.M., Cassidy T.W., Varga G.A. Effects of rumen-protected choline and dry propylene glycol on feed intake and blood parameters for Holstein dairy cows in early lactation. J. Dairy Sci. 2009;92:2729–2736. doi: 10.3168/jds.2008-1299. [DOI] [PubMed] [Google Scholar]
- Curtis C.R., Hollis M.E., Sniffen C.J., Smith R.D., Kronfeld D.S. Path analysis of dry period nutrition, postpartum metabolic and reproductive disorders, and mastitis in Holstein cows. J. Dairy Sci. 1985;68:2347–2360. doi: 10.3168/jds.S0022-0302(85)81109-7. [DOI] [PubMed] [Google Scholar]
- DeFrain J.M., Hippen A.R., Kalscheur K.F., Jardon P.W. Feeding glycerol to transition dairy cows: Effects on blood metabolites and lactation performance. J. Dairy Sci. 2004;87:4195–4206. doi: 10.3168/jds.S0022-0302(04)73564-X. [DOI] [PubMed] [Google Scholar]
- Drackley J.K., LaCount D.W., Elliott J.P., Klusmeyer T.H., Overton T.R., Clark J.H., Blum S.A. Supplemental fat and nicotinic acid for Holstein cows during an entire lactation. J. Dairy Sci. 1998;81:201–214. doi: 10.3168/jds.S0022-0302(98)75567-5. [DOI] [PubMed] [Google Scholar]
- Duffield T.F., Rabiee A.R., Lean I.J. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 1. Metabolic effects. J. Dairy Sci. 2008;91:1334–1346. doi: 10.3168/jds.2007-0607. [DOI] [PubMed] [Google Scholar]
- EMEA. 1999. The European Agency for the Evaluation of Medicinal Products. Veterinary Medicines and Information Technology Unit. EMEA/MRL/630/99-FINAL, p 1–3. EMEA, London, UK.
- EMEA. 2000. The European Agency for the Evaluation of Medicinal Products. Veterinary Medicines and Information Technology Unit. EMEA/MRL/734/00-FINAL, p 1–2. EMEA, London, UK.
- Flasshoff, F. H. 1974. Clinical and chemical blood serum investigations in cattle and treatment studies with ornithine-aspartate-product HMV 20 and with Catosal for the reduction of fertility and health disorders. PhD thesis. Tierärztliche Hochschule, Hannover, Germany.
- Fürll M. Vet. Med. Habil.-Schrift.; Leipzig, Germany: 1989. Ätiologie, Pathogenese, Diagnostik und medikamentelle Beeinflussung von Leberschäden beim Rind. [Google Scholar]
- Fürll M., Jäckel F. Effects of glucocorticoids on parameters of lipid metabolism, hepatic metabolism, haematological parameters and milk yield in high-yielding cows in early lactation. Berl. Munch. Tierarztl. Wochenschr. 2005;118:247–254. [PubMed] [Google Scholar]
- Fürll M., Wittek T., Gengenbach S., Schmidt B. Effekte einer präoperativen Applikation von Butafosfan und Cyanocobalamin auf Rekonvaleszenz, klinisch-chemische Parameter, antioxidativen Stoffwechsel und postoperative Labmagenentleerung bei Kühen mit Dislocatio abomasi. Tierärztl. Prax. 2006;34(G):351–356. [Google Scholar]
- Gerloff B.J., Herdt T.H., Emery R.S. Relationship of hepatic lipidosis to health and performance in dairy cattle. J. Am. Vet. Med. Assoc. 1986;188:845–850. [PubMed] [Google Scholar]
- Girard C.L., Matte J.J. Effects of intramuscular injections of vitamin B12 on lactation performance of dairy cows fed dietary supplements of folic acid and rumen-protected methionine. J. Dairy Sci. 2005;88:671–676. doi: 10.3168/jds.S0022-0302(05)72731-4. [DOI] [PubMed] [Google Scholar]
- Graulet B., Matte J.J., Desrochers A., Doepel L., Palin M.-F., Girard C.L. Effects of dietary supplements of folic acid and vitamin B12 on metabolism of dairy cows in early lactation. J. Dairy Sci. 2007;90:3442–3455. doi: 10.3168/jds.2006-718. [DOI] [PubMed] [Google Scholar]
- Grünberg W., Constable P.D., Schroder U., Staufenbiel R., Morin D.E., Rohn M. Phosphorus homeostasis in dairy cows with abomasal displacement or abomasal volvulus. J. Vet. Intern. Med. 2005;19:894–898. doi: 10.1892/0891-6640(2005)19[894:phidcw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grummer R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008;176:10–20. doi: 10.1016/j.tvjl.2007.12.033. (Review) [DOI] [PubMed] [Google Scholar]
- Grünberg W., Staufenbiel R., Constable P.D., Dann H.M., Morin D.E., Drackley J.K. Liver phosphorus content in Holstein-Friesian cows during the transition period. J. Dairy Sci. 2009;92:2106–2117. doi: 10.3168/jds.2008-1897. [DOI] [PubMed] [Google Scholar]
- Hansel A., Fuhrmann H., Sallman H.P. Intravenous infusion of volatile fatty acids as a loading test for the evaluation of possible effects of butafosfane on the energy metabolism of cattle. Berl. Munch. Tierarztl. Wochenschr. 1992;105:361–366. [PubMed] [Google Scholar]
- Kristensen N.B., Raun B.M. Ruminal and intermediary metabolism of propylene glycol in lactating Holstein cows. J. Dairy Sci. 2007;90:4707–4717. doi: 10.3168/jds.2007-0295. [DOI] [PubMed] [Google Scholar]
- LaCount D.W., Drackley J.K., Weigel D.J. Responses of dairy cows during early lactation to ruminal or abomasal administration of L-carnitine. J. Dairy Sci. 1995;78:1824–1836. doi: 10.3168/jds.S0022-0302(95)76807-2. [DOI] [PubMed] [Google Scholar]
- Leroy J.L., Vanholder T., Opsomer G., Van Soom A., De Kruifa A. The in vitro development of bovine oocytes after maturation in glucose and beta-hydroxybutyrate concentrations associated with negative energy balance in dairy cows. Reprod. Domest. Anim. 2006;41:119–123. doi: 10.1111/j.1439-0531.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- Lohr B., Brunner B., Kanowitz H. Clinical efficacy of Catosal in the treatment of ketosis in cows with left abomasal displacement. Tierarztl. Umsch. 2006;61:187–190. [Google Scholar]
- Moallem U., Katz M., Arieli A., Lehrer H. Effects of peripartum propylene glycol or fats differing in fatty acid profiles on feed intake, production, and plasma metabolites in dairy cows. J. Dairy Sci. 2007;90:3846–3856. doi: 10.3168/jds.2007-0092. [DOI] [PubMed] [Google Scholar]
- Morrow D.A. Fat cow syndrome. J. Dairy Sci. 1976;59:1625–1629. doi: 10.3168/jds.S0022-0302(76)84415-3. [DOI] [PubMed] [Google Scholar]
- Mosley S.A., Mosley E.E., Hatch B., Szasz J.I., Corato A., Zacharias N., Howes D., McGuire M.A. Effect of varying levels of fatty acids from palm oil on feed intake and milk production in Holstein cows. J. Dairy Sci. 2007;90:987–993. doi: 10.3168/jds.S0022-0302(07)71583-7. [DOI] [PubMed] [Google Scholar]
- Mykkänen J., Korpela H. Serum vitamin B12 levels in dairy cows before and after parturition. Zentralbl. Veterinarmed. A. 1981;28:526–528. doi: 10.1111/j.1439-0442.1981.tb01222.x. [DOI] [PubMed] [Google Scholar]
- Nafikov R.A., Ametaj B.N., Bobe G., Koehler K.J., Young J.W., Beitz D.C. Prevention of fatty liver in transition dairy cows by subcutaneous injections of glucagon. J. Dairy Sci. 2006;89:1533–1545. doi: 10.3168/jds.S0022-0302(06)72221-4. [DOI] [PubMed] [Google Scholar]
- Palmer C.R. “Metaphylaxis” in post-partum conditions in dairy cows with butaphosphone: A trial under South African conditions. J. S. Afr. Vet. Assoc. 1980;51:239–242. [PubMed] [Google Scholar]
- Pires J.A., Pescara J.B., Grummer R.R. Reduction of plasma NEFA concentration by nicotinic acid enhances the response to insulin in feed-restricted Holstein cows. J. Dairy Sci. 2007;90:4635–4642. doi: 10.3168/jds.2007-0146. [DOI] [PubMed] [Google Scholar]
- Preynat A., Lapierre H., Thivierge M.C., Palin M.F., Matte J.J., Desrochers A., Girard C.L. Effects of supplements of folic acid, vitamin B12, and rumen-protected methionine on whole body metabolism of methionine and glucose in lactating dairy cows. J. Dairy Sci. 2009;92:677–689. doi: 10.3168/jds.2008-1525. [DOI] [PubMed] [Google Scholar]
- Rollin E., Berghaus R.D., Rapnicki P., Godden S.M., Overton M.W. The effect of injectable butaphosphan and cyanocobalamin on postpartum serum β-hydroxybutyrate, calcium, and phosphorus concentrations in dairy cattle. J. Dairy Sci. 2010;93:978–987. doi: 10.3168/jds.2009-2508. [DOI] [PubMed] [Google Scholar]
- Santschi D.E., Berthiaume R., Matte J.J., Mustafa A.F., Girard C.L. Fate of supplementary B-vitamins in the gastrointestinal tract of dairy cows. J. Dairy Sci. 2005;88:2043–2054. doi: 10.3168/jds.S0022-0302(05)72881-2. [DOI] [PubMed] [Google Scholar]
- Schuh, R. 1994. Investigations on the efficacy of butafosfan in the prevention of metabolic disorders in dairy cows in the peri-partal period. DVM thesis. Justus Liebig University, Giessen, Germany.
- Schulz K.F., Grimes D.A. Generation of allocation sequences in randomised trials: Chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- Selberg K.T., Lowe A.C., Staples C.R., Luchini N.D., Badinga L. Production and metabolic responses of periparturient Holstein cows to dietary conjugated linoleic acid and trans-octadecenoic acids. J. Dairy Sci. 2004;87:158–168. doi: 10.3168/jds.S0022-0302(04)73153-7. [DOI] [PubMed] [Google Scholar]
- Sommer H., Marx D., Starker G. Reduction of cattle fertility disorders using Catosal during metaphylaxis. Dtsch. Tierarztl. Wochenschr. 1971;78:593–597. [PubMed] [Google Scholar]
- Steinberg W., Klünter A.-M. Einsatz von B-Vitaminen in der Wiederkäuerfütterung. In: Schubert R., Flachowsky G., Bitsch R., editors. Vitamine und Zusatzstoffe in der Ernährung von Mensch und Tier. 5. Symposium, Jena/Thüringen. Buch- und Kunstdruckerei Kessler GmbH; Weimar, Germany: 1995. [Google Scholar]
- van der Staay F.J., de Groot J., van Reenen C.G., Hoving-Bolink A.H., Schuurman T., Schmidt B.H. Effects of butafosfan on salivary cortisol and behavioral response to social stress in piglets. J. Vet. Pharmacol. Ther. 2007;30:410–416. doi: 10.1111/j.1365-2885.2007.00884.x. [DOI] [PubMed] [Google Scholar]