Abstract
The ferret is an established animal model for a number of human respiratory viral infections, such as influenza virus and more recently, Ebola virus. However, a paucity of immunological reagents has hampered the study of cellular immune responses. Here we describe the development and characterisation of a novel monoclonal antibody (mAb) against the ferret CD4 antigen and the characterisation of ferret CD4 T lymphocytes. Recombinant production and purification of the ferret CD4 ectodomain soluble protein allowed hybridoma generation and the generation of a mAb (FeCD4) showing strong binding to ferret CD4 protein and lymphoid cells by flow cytometry. FeCD4 bound to its cognate antigen post-fixation with paraformaldehyde (PFA) which is routinely used to inactivate highly pathogenic viruses. We have also used FeCD4 in conjunction with other immune cell markers to characterise ferret T cells in both primary and secondary lymphoid organs. In summary, we have developed an important reagent for the study of cellular immunological responses in the ferret model of infectious disease.
Keywords: T cells, Ferret, Antibody, CD4
Highlights
-
•
First isolation and functional characterisation of ferret lymphocytes
-
•
Ferret Lymphocytes display similar distribution to laboratory animals and humans.
-
•
Novel CD4 mAb detects its antigen following PFA fixation.
-
•
Enhancement of infectious disease immunology through use of ferret model
1. Introduction
The ferret is a well-established small animal model for the study of the infectivity, pathogenesis and transmission of both human and avian influenza viruses (Belser et al., 2011). In addition, the recent emergence of a number of novel zoonotic viral pathogens with the potential for causing fatal disease in human hosts has extended the biomedical significance of this species. Infection of ferrets with severe acute respiratory coronavirus (SARS-CoV) recapitulates a number of the clinical and pathophysiological signs observed in human infections, including viral replication in the upper and lower respiratory tract and significant lung damage (Chu et al., 2008, See et al., 2008, Martina et al., 2003). The related paramyxoviruses, Hendra and Nipah, exhibit a broad host range and cause acute respiratory infection and severe encephalitis associated with high mortality in humans (Vigant and Lee, 2011). Ferrets infected with either virus exhibit a disease pathology very similar to that observed in humans, characterised by widespread vasculitis, and severe respiratory and neurological disease (Bossart et al., 2009, Pallister et al., 2011). More recently, the ferret has been shown to recapitulate signs of human disease signs when infected with different species of ebolavirus (Cross et al., 2016), further demonstrating that the ferret provides a robust model for the evaluation of preventative and passive immune protection from infection.
Despite the importance of the ferret as a model for human viral diseases, research using the ferret has been hampered by a lack of ferret-specific immunological reagents to explore the cellular immune system. To date, the identification of monoclonal antibodies (mAbs) that recognise ferret immune cell markers and cytokines has relied largely on screens of antibodies in other species that cross-react with the ferret counterpart (Martel and Aasted, 2009, Rutigliano et al., 2008). While this approach has been successful in identifying antibodies that recognise, for example, CD8 (Rutigliano et al., 2008), gaps remain in the repertoire of available reagents. A number of studies have been published that describe the use of ferret-specific CD4 mAbs to monitor changes in the level of CD4 T lymphocytes in ferrets in response to different viral infections (Cheng et al., 2013, Music et al., 2016, Music et al., 2014, Xu et al., 2013). Most recently, DiPiazza et al. (2016) used both CD4 and CD8 specific reagents to look at changes in the antigen-specific T cell repertoire following influenza infection in ferrets. As the ferret is becoming a commonly used model for highly pathogenic virus studies such as Ebola virus, further characterisation with regards to phenotypic and functional aspects of the ferret CD4 T cell population are needed. Furthermore, reagents that can detect the antigen following virus inactivation are critical. In the current study, we describe the derivation and characterisation of a ferret CD4 specific mAb that is well-suited for studies involving infectious material.
2. Materials and methods
2.1. Animals and ethics
All animal studies were approved by the CSIRO Australian Animal Health Laboratory Animal Ethics Committee and conducted following the Australian National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Scientific Purposes guidelines for housing and care of laboratory animals. Ferrets aged 12–18 months were euthanized and organs removed aseptically.
2.2. Protein structure and homology analysis
CLC Main Workbench Version 7.0.2 was used to compare protein and nucleic acid sequences derived from National Centre for Biotechnology Information (NCBI), UniprotKB and Ensembl. The sequences for mouse (AAC36010.1), human (AAB51309.1), ferret (ABS50090.1), dog (AAB02295.1), pig (ANE20437.1) and chicken (ABA55042.1) were converted to FASTA format before analysing the data. The three-dimensional (3D) CD4 models were constructed by using the automated comparative protein modelling server SWISS-MODEL (http://swiismodel.expasy.org).
2.3. Vector assembly
A DNA template encoding the signal peptide and extracellular domain of ferret CD4 (amino acids 1–400; NCBI RefSeq EF492055.1), together with a C-terminal Flag tag (DYKDDDDK), was optimised for expression in human cell lines and synthesised by GeneArt AG (Life Technologies). The coding region insert was isolated and sub-cloned into the mammalian expression vector, pEE6.4 (Lonza).
2.4. Cell culture, transient transfection and purification of soluble CD4
Suspension-adapted cultures of FreeStyle-293 cells (Life Technologies) were grown in Freestyle 293 Expression Medium (Life Technologies) supplemented with Glutamax-I. Scale-up transient transfections were performed on a 1 L culture of cells (2 × 106 cells/mL) in a 3 L flask using linear polyethylenimine (PEI, Polysciences Inc.) according to a published protocol (Tom et al., 2008). The culture was harvested after seven days (cell viability > 50%), clarified by centrifugation at 400g and filtered. Soluble, recombinant CD4 was purified from 1.1 L of conditioned medium by immunoaffinity chromatography followed by size exclusion chromatography through Superdex™ S200 (GE Healthcare) as previously described (Elleman et al., 2001). The resulting peak fractions were concentrated and sterile-filtered.
2.5. Derivation of hybridomas
The production of mAbs was undertaken at the Monash Antibody Technology Facility, Clayton, Australia. Briefly, CD1 mice were immunised intraperitoneally with ferret CD4 protein (10 μg/injection) in a water-in-oil emulsion (Sigma) containing CpG, three times at two week intervals. Two weeks after the third immunisation a serum sample was taken and the antibody titre assessed using a conventional ELISA format using antigen-coated wells. Once a serum titre of three-fold higher than background (pre-bleed) was reached, a final boost immunisation was given followed four days prior to euthanasia and splenectomy. The spleen cells were homogenised into a single cell suspension between two frosted glass slides and 100 × 106 spleen cells were mixed with 25 × 106 SP2/0 cells. These cells were washed in DMEM and 1 mL of polyethylene glycol was added drop-wise over a one minute period follow by additional washes in DMEM. The cells were plated into 20 × 96 well plates and allowed to divide for two weeks. At this point the supernatant from each well was tested for reactivity against the immunogen by protein microarray using antigen-coated slides and further confirmed by ELISA, and positive binders were expanded, subcloned until clonal and frozen. The production and purification of the FeCD4 was undertaken in pleated-surface roller bottles in Hybridoma-serum free media (Gibco) supplemented with 1% heat-inactivated FBS at 37 °C for 10 days. The resultant medium was filtered through a 0.22 μm filter and applied to a Protein A Sepharose column (Fast Flow 4; GE) and antibody fraction eluted with 50 mM glycine, pH 3.0, into tubes containing 0.2 elution volumes of 1 M Tris-HCl, pH 8.0. Antibody fractions were exchanged into phosphate-buffered saline (PBS) and concentrated using Amicon Ultra units (Millipore).
2.6. Fluorescence-activated cell scanning
Spleen, thymus and lymph node were excised from euthanized ferrets, cleaned of any connective tissue and mechanically digested in cold FACS buffer (2% (v/v) FCS, 0.02% (v/v) NaN3 in PBS) to produce a single suspension. Mechanical digestion was achieved by pressing the tissue through a 70 μm sieve (BD Biosciences) using the plunger of a 10 mL syringe. Red blood cells were removed from the spleen by Lymphoprep density gradient (Stemcell Technologies) centrifugation at 1000g for 20 min with no brake. One million cells were stained with 0.1 μg of anti-ferret CD4, washed in FACS buffer and resuspended in 150 μL of FACS buffer. Additional antibodies used for phenotyping included CD8 (OKT8), CD81 (JS081), CD11b (M1/70) (BD Biosciences), MHC class II (CAT82a) (Kingfisher) and anti-ferret Ig (H + L) (Rockland). Where required an anti-IgG2a or anti-IgG1 secondaries were used (Invitrogen). For fixation cells were resuspended in 200 μL of 4% paraformaldehyde (PFA) in PBS. Data was acquired using a BD FACS LSRII flow cytometer (BD Biosciences) equipped with 405, 488, and 633 nm excitation lasers in conjunction with FACS Diva acquisition software (BD Biosciences). Data analysis was performed using FlowLogic FCS analysis software (Inivai Technologies).
2.7. ELISpot
Lymph node (LN) cells (1.5 × 108 cells) from three separate ferrets were stained with FeCD4 for 30 min at 4 °C. The cells were washed with 10 mL cold PBS + 2% FCS and centrifuged at 400g for 5 min. The supernatant was decanted and cells were resuspended in 2 mL PBS/FCS and 150 μL of MACS anti-mouse IgG beads added and incubated at 4 °C for 15 min. The cells were washed again and passed over a MACS LS column (Miltenyi) according to manufacturer's instructions. The CD4 cells were collected and remaining cells stained with CD8 (clone OKT8) and previous steps repeated. The cells were then plated in a 96 well Ferret IFNγ ELISpot plate (Mabtech) at a density of 2.5 × 104 cells/well in 100 μL with or without ConA (10 μg/mL) and the cells cultured for 48 h. The ELISpots were then developed and counted as per the manufacturer's instructions.
3. Results and discussion
3.1. Ferret CD4 homology and predicted structure
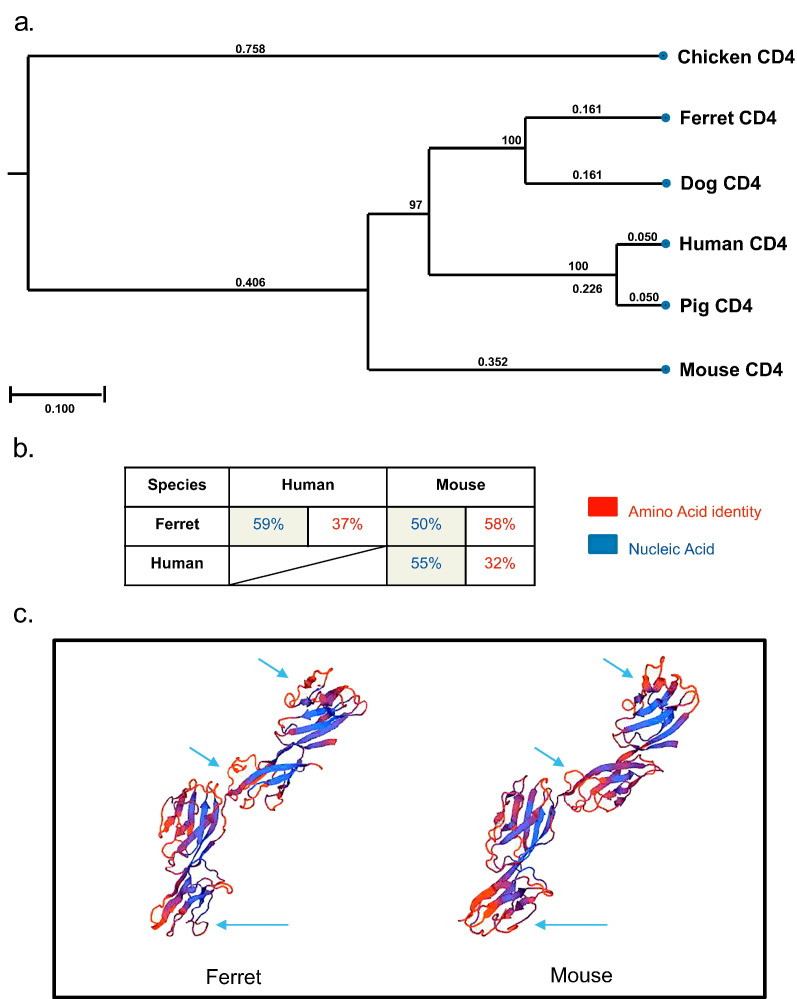
Analysis of CD4 protein sequences shows that of the proteins compared, the ferret CD4 protein is most closely related to dog followed by human and pig (Fig. 1a). As the majority of CD4-specific antibodies are more commonly available for human and mouse we investigated the conservation of ferret with these two species. Nucleic acid level analysis showed the human and ferret CD4 genes were 59% identical, whereas mouse and ferret were 50%. Intriguingly, at the amino acid level, a more important measure for cross-reactive antibody binding, mouse and ferret displayed 58% identity compared to 37% between human and ferret. These low levels of cross-species homology may explain why human and mouse antibodies are not cross-reactive with the ferret CD4 protein.
Fig. 1.
Ferret CD4 protein has a higher amino acid identity to mouse CD4 than human. Phylogenetic tree shows the homology of the CD4 amino acid sequences between ferret and other species (a). The table shows the percent identity of CD4 between ferret, mouse and human amino acid and nucleic acid sequences (b). The 3D predicted structure of mouse and ferret CD4 molecule, smooth curved lines depict polypeptide backbones, whereas the β-strands are displayed as thick coloured arrows and indicate the direction of the polypeptide chain (c). Arrows indicate some of the possible surface difference between the two molecules.
The CD4 protein is well-described as a co-receptor in the T cell receptor (TCR) complex and is a member of the immunoglobulin superfamily, containing four tandem Ig-like domains (Clark et al., 1987). We generated a predicted 3D structure for the ferret CD4 molecule and compared it to that of a mouse CD4 3D structure. Based on 3D predictions, the amino acid identity confers many similar structural features including the four Ig-like domains (Fig. 1c).
3.2. Generation of recombinant ferret CD4 and proteomic analysis
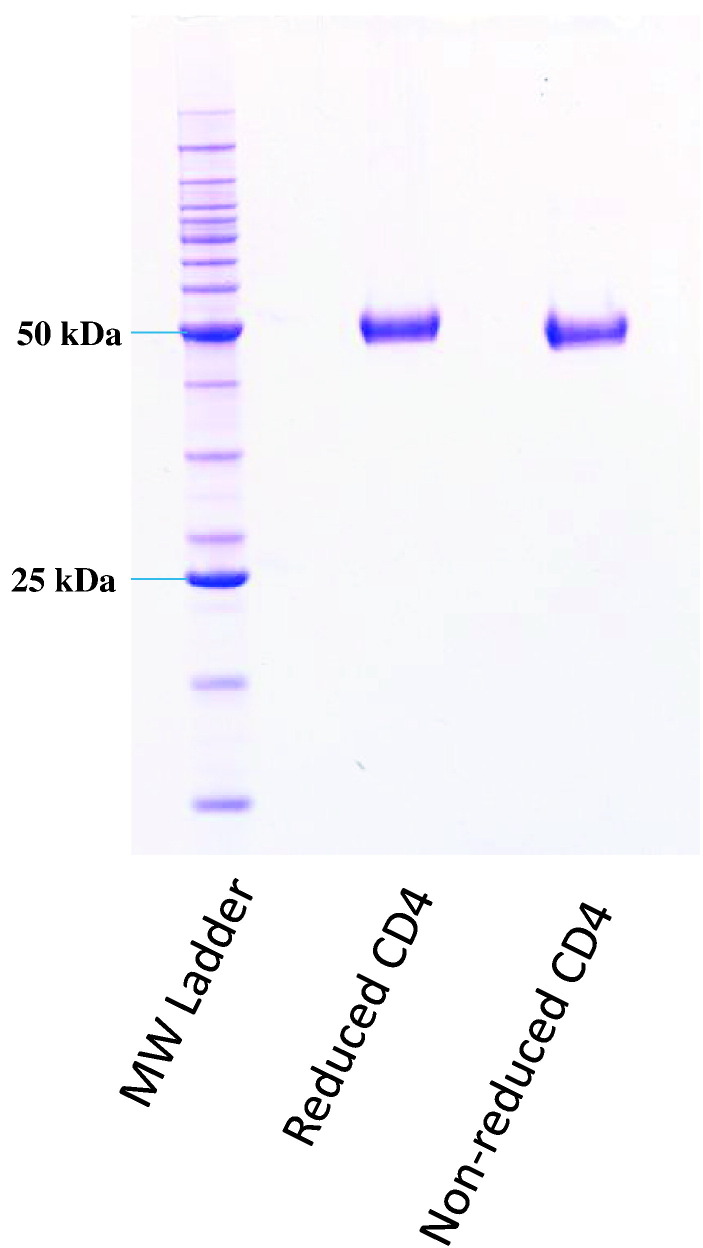
A ferret CD4 cDNA clone was used as a template for the amplification and cloning of a Flag-tagged ectodomain coding region corresponding to residues 1–400, including the native signal peptide. Transient expression resulted in the biosynthesis and secretion of soluble ferret CD4 ectodomain (data not shown). A subsequent transfection of a 1 L culture of suspension-adapted FreeStyle™-293 cells allowed the purification of a total of 4 mg of soluble CD4 with a molecular weight of approximately 50 kDa (Fig. 2 ), which migrated as a monomer upon size exclusion chromatography (data not shown). This size would indicate a contribution of approximately 8 kDa by glycosylation. Glycosylation is an important feature of the CD4 molecule, impacting localisation to the cell membrane and protein folding (Tifft et al., 1992). N-terminal amino acid sequencing performed using Edman degradation validated the predicted mature N-terminus (REVVLGKVGD; data not shown).
Fig. 2.
Generation of recombinant ferret CD4. The SDS-PAGE gel shows both the reduced and non-reduced forms of the purified ferret CD4 protein. The molecular weight (MW) ladder shows relative size.
3.3. Hybridoma generation
We (data not shown) and others (Rutigliano et al., 2008) have demonstrated that mAbs generated against mouse CD4 do not cross react with native ferret CD4 on the surface of lymphocytes. Furthermore, as the ferret is employed as a model for infectious disease research, where virus inactivation methods often destroy or block antibody binding epitopes, an anti-ferret CD4 antibody that is compatible with post-cellular fixation protocols would be a valuable resource. Therefore, we decided to generate a novel mAb against ferret CD4 that would have specific use on paraformaldehyde (PFA)-fixed samples by flow cytometry, where PFA fixation is commonly employed to inactivate infectious material (Cross et al., 2016).
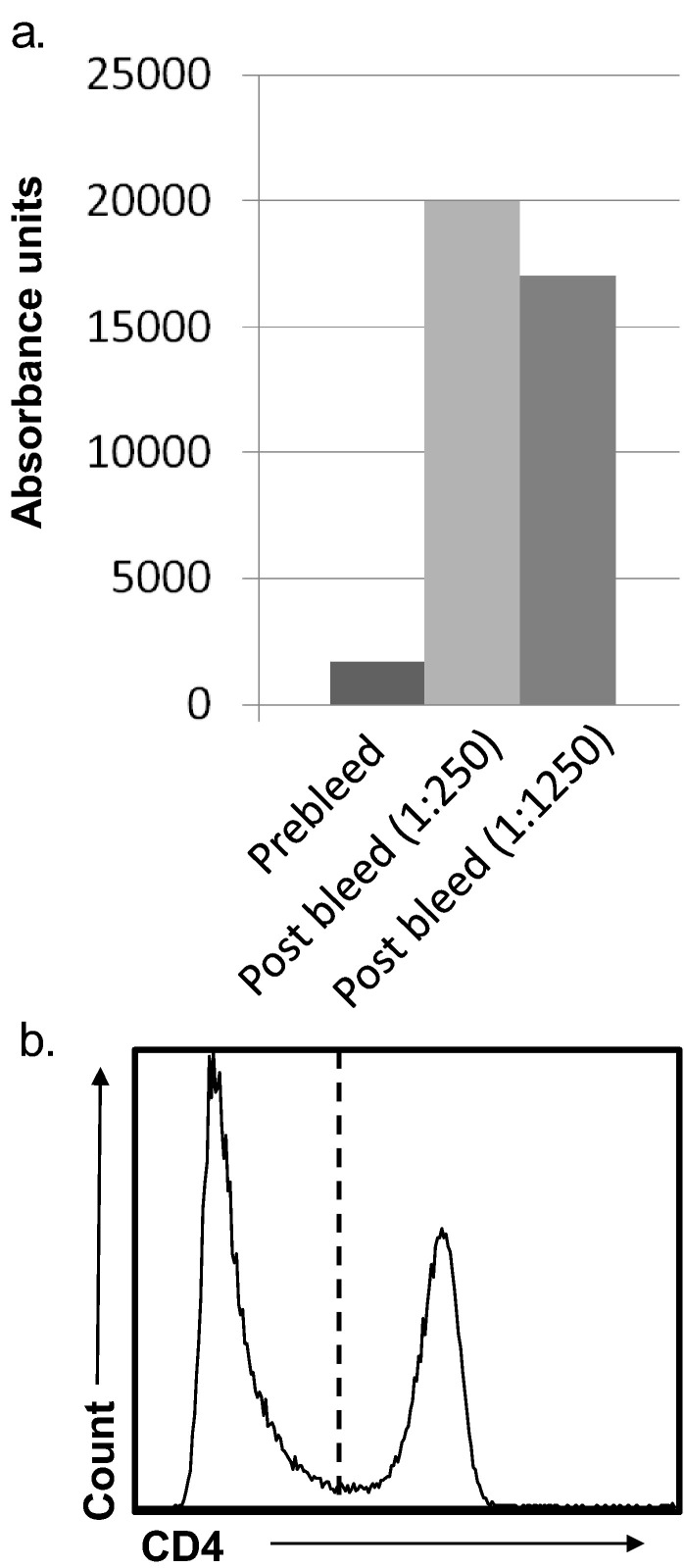
Following three immunisations, mice demonstrated a high anti-CD4 serum titre by ELISA (Fig. 3a). We then generated > 1000 hybridoma clones, many of which tested positive by ELISA against the recombinant CD4 protein (data not shown). We screened a number of these by flow cytometry against a single-cell suspension of ferret lymph node; one antibody, designated FeCD4, which bound 35% of cells, was selected for further study (Fig. 3b). Similar proportions of CD4+ cells are reported in mouse lymph node (Mouchacca et al., 2015). The proportion of cellular expression of CD4 in primary and secondary lymphoid organs is similar to human and mouse (Lagresle-Peyrou et al., 2006).
Fig. 3.
A novel anti-ferret CD4 mAb binds LN cells. The bar graph shows the serum ELISA response of a mouse immunised with rfCD4 as expressed as absorbance units. The post immunisation serum (1:250 and 1:1250 dilution) is compared with a pre-immunisation serum (1:250 dilution).
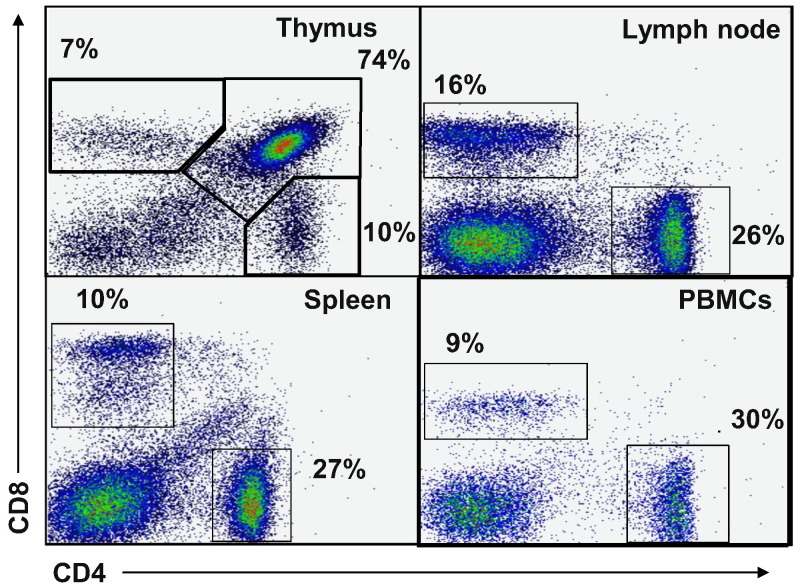
We looked at the expression of the CD4 antigen in multiple lymphoid tissues including blood, spleen, lymph node and thymus. This served a dual purpose of demonstrating that the FeCD4 binds CD4 T cells, in addition to providing a profile of the ferret T cell population in the ferret immune compartment. The expression of the CD4 protein when analysed with CD8 in many species displays a very distinct staining pattern in the thymus, comprised predominantly of double-positive immature thymocytes (Chidgey et al., 2008). In the ferret thymus, the majority of the CD4 positive cells were also CD8 positive (74%), with the remaining 10% of CD4+ cells being CD8− and approximately 7% of the thymus being CD8 single-positive (Fig. 4 ). As might be expected, none of the remaining tissues (LN, spleen and blood) had significant numbers of CD4+ CD8+ cells but predominantly displayed CD4/8 single-positive profiles. The single-positive proportions of CD8 T cells were 16, 10 and 9% and the single-positive CD4 T cell proportions were 26, 27 and 30% for LN, spleen and PBMCs, respectively (data not shown). Interestingly, there was a population of CD8lowCD4− cells present in the spleen. The forward scatter (size) vs. side scatter (granularity) of these cells suggests that they may be monocytes (data not shown). This would not be unexpected, as many monocyte populations in humans and mice express CD8, including dendritic cells (Gibbings and Befus, 2009).
Fig. 4.
CD4 and CD8 immune cell subset in ferret lymphoid organs. The density plots show CD4 and CD8 antibody binding to ferret thymus, lymph node, spleen and PBMCs. Percentages indicate the indicate the proportion of cells within a gated area and are representative of n = 4.
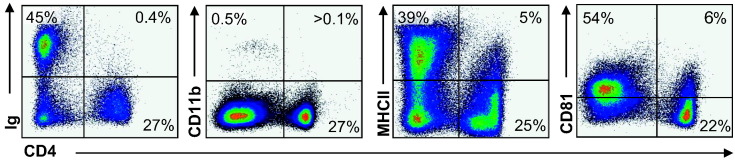
Immune cell phenotyping was also performed on ferret LN to further confirm the binding properties of FeCD4, but also to gain an understanding of different ferret immune cell subset phenotypes. We co-stained cells with the CD4 antibody and with anti-ferret Ig and cross-reactive anti-mouse CD11b, anti-bovine MHC class II and anti-human CD81 (Fig. 5 ). As expected, the anti-Ig (B cells) and the anti-CD11b antibodies bound a separate population of cells to that of the CD4 mAb. In the ferret LN we observed a very low proportion of CD11b positive cells, possibly due to mechanical not enzymatic digestion. Interestingly, a small subset (5%) of LN cells expressed both MHC class II and CD4. This is consistent with findings that many dendritic cells as well as some activated T cells from other mammals co-express these markers (Vremec et al., 2000), suggesting a possible mechanism for sub-setting antigen-presenting cells in the ferret, with the majority of the MHC class II cells being B cells in the LN. Additionally, we show here for the first time the ability for an anti-human CD81 mAb to cross-react with ferret LN cells. The majority of LN cells display low expression of CD81, as is expected as CD81 is well-known to be present on the surface of B cells. Interestingly, the majority of CD4 T cells in the ferret (22%) were negative for the CD81 marker, however a subset (6%) were positive for both CD4 and CD81. CD81 has been shown to be an important co-stimulator of T cells, in particular driving a Th2 function (Deng et al., 2000). Our data indicates a possible mechanism for distinguishing differentially activated CD4 T cells in the ferret.
Fig. 5.
MHC class II and CD81 subsets CD4+ cells in ferret LN. The dot plots show the antibody binding of the CD4 mAb in combination with anti-Ig, anti-CD11b, anti-MHCII and anti-CD81. Percentages indicate proportion of cells in that quadrant. Plots are representative of flow staining from three separate ferrets.
3.4. FeCD4 defines a subset of cells that produce IFNγ upon ConA activation
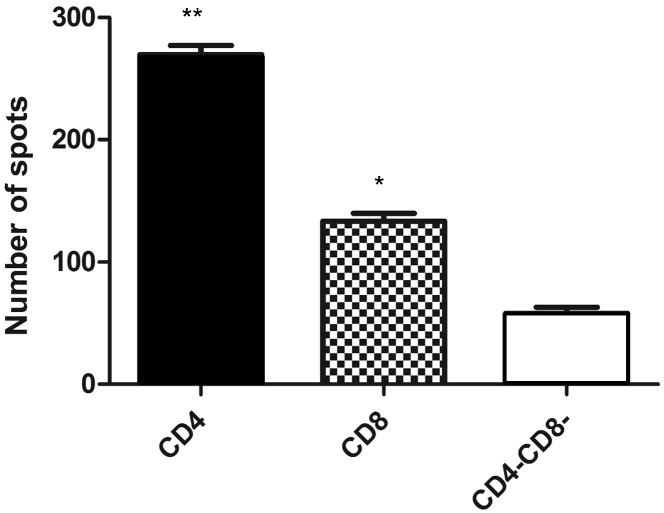
One of the key functional feature of T cells, including CD4 T cells, is their ability to respond to activation stimuli by production of IFNγ a critical Th1 cytokine that both acts on and is produced by T cells (Whitmire et al., 2005). In particular, T cells will produce IFNγ in response to a polyclonal stimulus such as Concanavalin A (ConA). Palacios (1982) demonstrated that ConA activation of CD4 T cells does not require MHCII interaction but rather directly stimulates receptors for activation, later shown to be TRPM2 and TRPC3 (Palacios, 1982, Pang et al., 2012). In Fig. 6 , we show that CD4 and CD8 T cells sorted from ferret LN, using the FeCD4 and OKT8 antibodies, respectively, are able to initiate an IFNγ response to ConA as measured by ELISpot. The ConA-responsive cells are present at significantly higher (p < 0.05) levels in the CD4+ and CD8+ compared to the CD4− CD8− cells from the same tissue.
Fig. 6.
Conventional ferret T cells have an increased ability to produce IFNγ compared to CD4− CD8− cells. The bar graph shows the number of IFNγ positive ELISpots for CD4+, CD8+ and CD4− CD8− cells from ferret lymph node per 25,000 cells. Results are from three independent biological samples (n = 3) and * = p < 0.05, ** = p < 0.01.
Ferrets are widely used to study human-adapted strains of influenza, as they possess a similar distribution of sialic acid α2,6 in the respiratory tract (Jayaraman et al., 2012) and more recently have been shown to lack N-glycolylneuraminic acid (Ng et al., 2014). Cell-mediated immunity has been shown to be of critical importance in influenza infections, however, to date, the majority of our understanding has come from mouse models and mouse-adapted strains of the virus (Thomas et al., 2006). This includes the priming of Th1 and Th2 cells to stimulate cytotoxic T cells and B cells responses (Justewicz et al., 1995) as well as CD4 T cell memory (Strutt et al., 2010). As it has been shown numerous times, the impact of using mouse-adapted virus in inbred strains of mice may convolute these findings. In order to study the ability of influenza A viruses to stimulate CD4 cells in a situation that more closely resemble that of human-adapted viruses, the use of FeCD4 mAb and the functional output of IFNγ will be highly valuable for future studies.
3.5. FeCD4 binds CD4 cells post-fixation with PFA
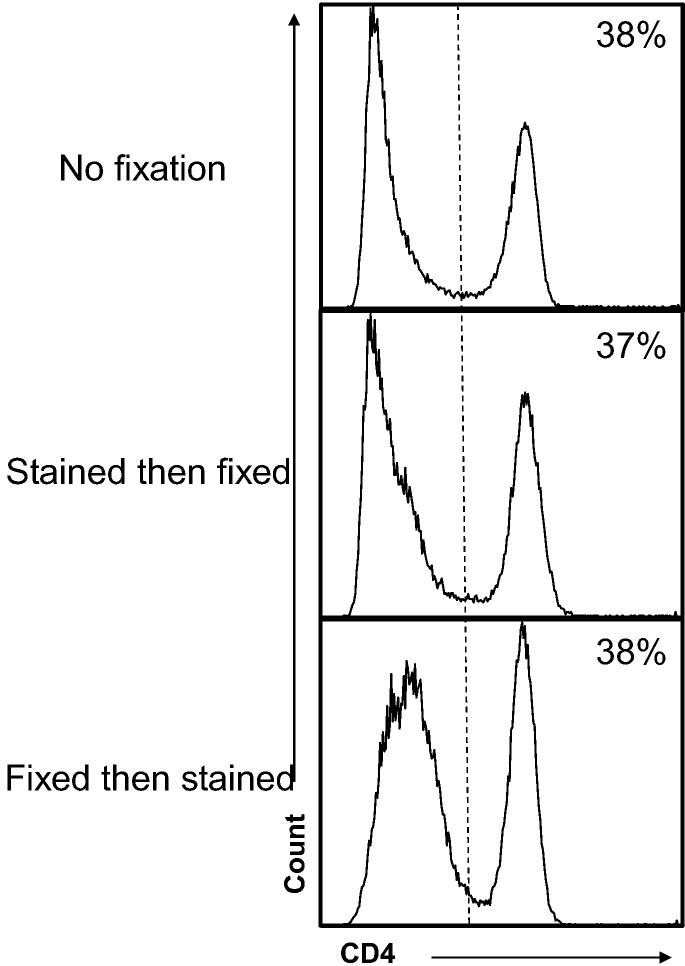
Many flow cytometry methods utilize PFA to fix cells following cell-surface staining, sometimes followed by permeabilization of the membrane for intracellular staining (Pockley et al., 2015). As such, we wanted to determine if our antibody could bind the CD4 antigen following fixation. Previous reports have used a range of 2–4% PFA to inactivate infectious material (Takada et al., 2007), therefore ferret LN cells were treated with 4% PFA for 2 h both before and after staining with the FeCD4 antibody. The mean fluorescence intensity (MFI) and percentage of cells stained against unfixed samples stained with the same mAb were compared. There was no significant difference between the proportion of cells stained with CD4 if the samples were stained before or after fixation (Fig. 7 ) when compared to no fixation. Furthermore, there was no difference in MFI of the CD4 positive cell population. As the ferret has been shown to be a suitable small animal model for a number of human viruses it may be pertinent to decontaminate (PFA fix) cells early in the experimental protocol. Recently, Cross et al. published the use of ferrets as a suitable model to study Ebola virus infection (Cross et al., 2016) without the need for virus adaptation as required for other small animal models (Cross et al., 2015). With BSL4 pathogens there in an increased difficulty in undertaking flow cytometric experiments and therefore our FeCD4 mAb, which is able to phenotype ferret immune cells post-decontamination, may be more convenient for studies of highly pathogenic viruses in ferrets.
Fig. 7.
CD4 antigen can be detected post PFA fixation. The histograms show CD4 expression on ferret lymph node with and without PFA fixation. Vertical lines indicate placement of negative control. Plots are representative of n = 3 and percentages indicate percent positive.
4. Conclusion
The use of ferrets as a reliable model for a number of pathogenic human viral infections emphasises the importance of understanding and characterising the ferret immune response (Carolan et al., 2015). In this paper we have built upon other recent publications that have sort to identify novel reagents for the ferret and have further characterised these reagents to ensure they are binding their cognate antigen. The FeCD4 mAb will now serve as a ferret-specific reagent to allow detailed immunological studies of the response to viral infections such as Ebola and Influenza. The fact that FeCD4 can recognise fixed antigen enhances the versatility of this reagent for use in circumstances that involve biohazardous entities.
Conflicts of interest
The authors declare there are no conflicts of interest.
References
- Belser J.A., Katz J.M., Tumpey T.M. The ferret as a model organism to study influenza A virus infection. Dis. Model. Mech. 2011;4:575–579. doi: 10.1242/dmm.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart K.N., Zhu Z., Middleton D., Klippel J., Crameri G., Bingham J., McEachern J.A., Green D., Hancock T.J., Chan Y.P., Hickey A.C., Dimitrov D.S., Wang L.F., Broder C.C. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolan L.A., Rockman S., Borg K., Guarnaccia T., Reading P., Mosse J., Kelso A., Barr I., Laurie K.L. Characterization of the localized immune response in the respiratory tract of ferrets following infection with influenza A and B viruses. J. Virol. 2015;90:2838–2848. doi: 10.1128/JVI.02797-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Zengel J.R., Suguitan A.L., Jr., Xu Q., Wang W., Lin J., Jin H. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J. Infect. Dis. 2013;208:594–602. doi: 10.1093/infdis/jit207. [DOI] [PubMed] [Google Scholar]
- Chidgey A.P., Layton D., Trounson A., Boyd R.L. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330–337. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- Chu Y.K., Ali G.D., Jia F., Li Q., Kelvin D., Couch R.C., Harrod K.S., Hutt J.A., Cameron C., Weiss S.R., Jonsson C.B. The SARS-CoV ferret model in an infection-challenge study. Virology. 2008;374:151–163. doi: 10.1016/j.virol.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.J., Jefferies W.A., Barclay A.N., Gagnon J., Williams A.F. Peptide and nucleotide sequences of rat CD4 (W3/25) antigen: Evidence for derivation from a structure with four immunoglobulin-related domains. Proc. Natl. Acad. Sci. U. S. A. 1987;84:1649–1653. doi: 10.1073/pnas.84.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R.W., Fenton K.A., Geisbert J.B., Mire C.E., Geisbert T.W. Modeling the disease course of zaire ebolavirus infection in the outbred guinea pig. J. Infect. Dis. 2015;212(Suppl. 2):S305–S315. doi: 10.1093/infdis/jiv237. [DOI] [PubMed] [Google Scholar]
- Cross R.W., Mire C.E., Borisevich V., Geisbert J.B., Fenton K.A., Geisbert T.W. The domestic ferret (Mustela putorius furo) as a lethal infection model for 3 species of ebolavirus. J. Infect. Dis. 2016;214:565–569. doi: 10.1093/infdis/jiw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Yeung V.P., Tsitoura D., DeKruyff R.H., Umetsu D.T., Levy S. Allergen-induced airway hyperreactivity is diminished in CD81-deficient mice. J. Immunol. 2000;165:5054–5061. doi: 10.4049/jimmunol.165.9.5054. [DOI] [PubMed] [Google Scholar]
- DiPiazza A., Richards K., Batarse F., Lockard L., Zeng H., Garcia-Sastre A., Albrecht R.A., Sant A.J. Flow cytometric and cytokine ELISpot approaches to characterize the cell-mediated immune response in ferrets following influenza virus infection. J. Virol. 2016;90:7991–8004. doi: 10.1128/JVI.01001-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T.C., Domagala T., McKern N.M., Nerrie M., Lonnqvist B., Adams T.E., Lewis J., Lovrecz G.O., Hoyne P.A., Richards K.M., Howlett G.J., Rothacker J., Jorissen R.N., Lou M., Garrett T.P., Burgess A.W., Nice E.C., Ward C.W. Identification of a determinant of epidermal growth factor receptor ligand-binding specificity using a truncated, high-affinity form of the ectodomain. Biochemistry. 2001;40:8930–8939. doi: 10.1021/bi010037b. [DOI] [PubMed] [Google Scholar]
- Gibbings D., Befus A.D. CD4 and CD8: an inside-out coreceptor model for innate immune cells. J. Leukoc. Biol. 2009;86:251–259. doi: 10.1189/jlb.0109040. [DOI] [PubMed] [Google Scholar]
- Jayaraman A., Chandrasekaran A., Viswanathan K., Raman R., Fox J.G., Sasisekharan R. Decoding the distribution of glycan receptors for human-adapted influenza A viruses in ferret respiratory tract. PLoS One. 2012;7 doi: 10.1371/journal.pone.0027517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justewicz D.M., Doherty P.C., Webster R.G. The B-cell response in lymphoid tissue of mice immunized with various antigenic forms of the influenza virus hemagglutinin. J. Virol. 1995;69:5414–5421. doi: 10.1128/jvi.69.9.5414-5421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagresle-Peyrou C., Yates F., Malassis-Seris M., Hue C., Morillon E., Garrigue A., Liu A., Hajdari P., Stockholm D., Danos O., Lemercier B., Gougeon M.L., Rieux-Laucat F., de Villartay J.P., Fischer A., Cavazzana-Calvo M. Long-term immune reconstitution in RAG-1-deficient mice treated by retroviral gene therapy: a balance between efficiency and toxicity. Blood. 2006;107:63–72. doi: 10.1182/blood-2005-05-2032. [DOI] [PubMed] [Google Scholar]
- Martel C.J., Aasted B. Characterization of antibodies against ferret immunoglobulins, cytokines and CD markers. Vet. Immunol. Immunopathol. 2009;132:109–115. doi: 10.1016/j.vetimm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Martina B.E., Haagmans B.L., Kuiken T., Fouchier R.A., Rimmelzwaan G.F., Van Amerongen G., Peiris J.S., Lim W., Osterhaus A.D. Virology: SARS virus infection of cats and ferrets. Nature. 2003;425:915. doi: 10.1038/425915a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchacca P., Chasson L., Frick M., Foray C., Schmitt-Verhulst A.M., Boyer C. Visualization of granzyme B-expressing CD8 T cells during primary and secondary immune responses to Listeria monocytogenes. Immunology. 2015;145:24–33. doi: 10.1111/imm.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Music N., Reber A.J., Lipatov A.S., Kamal R.P., Blanchfield K., Wilson J.R., Donis R.O., Katz J.M., York I.A. Influenza vaccination accelerates recovery of ferrets from lymphopenia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Music N., Reber A.J., Kim J.H., York I.A. Peripheral leukocyte migration in ferrets in response to infection with seasonal influenza virus. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P.S., Bohm R., Hartley-Tassell L.E., Steen J.A., Wang H., Lukowski S.W., Hawthorne P.L., Trezise A.E., Coloe P.J., Grimmond S.M., Haselhorst T., von Itzstein M., Paton A.W., Paton J.C., Jennings M.P. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat. Commun. 2014;5:5750. doi: 10.1038/ncomms6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R. Concanavalin A triggers T lymphocytes by directly interacting with their receptors for activation. J. Immunol. 1982;128:337–342. [PubMed] [Google Scholar]
- Pallister J., Middleton D., Wang L.F., Klein R., Haining J., Robinson R., Yamada M., White J., Payne J., Feng Y.R., Chan Y.P., Broder C.C. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011;29:5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang B., Shin D.H., Park K.S., Huh Y.J., Woo J., Zhang Y.H., Kang T.M., Lee K.Y., Kim S.J. Differential pathways for calcium influx activated by concanavalin A and CD3 stimulation in Jurkat T cells. Arch. Eur. J. Physiol. 2012;463:309–318. doi: 10.1007/s00424-011-1039-x. [DOI] [PubMed] [Google Scholar]
- Pockley, A.G., Foulds, G.A., Oughton, J.A., Kerkvliet, N.I., Multhoff, G., 2015. Immune Cell Phenotyping Using Flow Cytometry. Current Protocols in Toxicology / Editorial Board, Mahin D. Maines 66, 18 18 11–18 18 34. [DOI] [PubMed]
- Rutigliano J.A., Doherty P.C., Franks J., Morris M.Y., Reynolds C., Thomas P.G. Screening monoclonal antibodies for cross-reactivity in the ferret model of influenza infection. J. Immunol. Methods. 2008;336:71–77. doi: 10.1016/j.jim.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See, R.H., Petric, M., Lawrence, D.J., Mok, C.P., Rowe, T., Zitzow, L.A., Karunakaran, K.P., Voss, T.G., Brunham, R.C., Gauldie, J., Finlay, B.B., Roper, R.L., 2008. Severe acute respiratory syndrome vaccine efficacy in ferrets: whole killed virus and adenovirus-vectored vaccines. J. Gen. Virol. 89, 2136–2146. [DOI] [PubMed]
- Strutt T.M., McKinstry K.K., Dibble J.P., Winchell C., Kuang Y., Curtis J.D., Huston G., Dutton R.W., Swain S.L. Memory CD4 + T cells induce innate responses independently of pathogen. Nat. Med. 2010;16:558–564. doi: 10.1038/nm.2142. (551p following 564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Ebihara H., Feldmann H., Geisbert T.W., Kawaoka Y. Epitopes required for antibody-dependent enhancement of Ebola virus infection. J. Infect. Dis. 2007;196(Suppl. 2):S347–S356. doi: 10.1086/520581. [DOI] [PubMed] [Google Scholar]
- Thomas P.G., Keating R., Hulse-Post D.J., Doherty P.C. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tifft C.J., Proia R.L., Camerini-Otero R.D. The folding and cell surface expression of CD4 requires glycosylation. J. Biol. Chem. 1992;267:3268–3273. [PubMed] [Google Scholar]
- Tom R., Bisson L., Durocher Y. 2008. Transfection of HEK293-EBNA1 Cells in Suspension with Linear PEI for Production of Recombinant Proteins. CSH Protoc 2008. (pdb prot4977) [DOI] [PubMed] [Google Scholar]
- Vigant F., Lee B. Hendra and Nipah infection: pathology, models and potential therapies. Infect. Disord. Drug Targets. 2011;11:315–336. doi: 10.2174/187152611795768097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D., Pooley J., Hochrein H., Wu L., Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Whitmire J.K., Tan J.T., Whitton J.L. Interferon-gamma acts directly on CD8 + T cells to increase their abundance during virus infection. J. Exp. Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Huang Z., Gao X., Michel F.J., Hirsch G., Hogan R.J., Sakamoto K., Ho W., Wu J., He B. Infection of mice, ferrets, and rhesus macaques with a clinical mumps virus isolate. J. Virol. 2013;87:8158–8168. doi: 10.1128/JVI.01028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]