Figure 1.
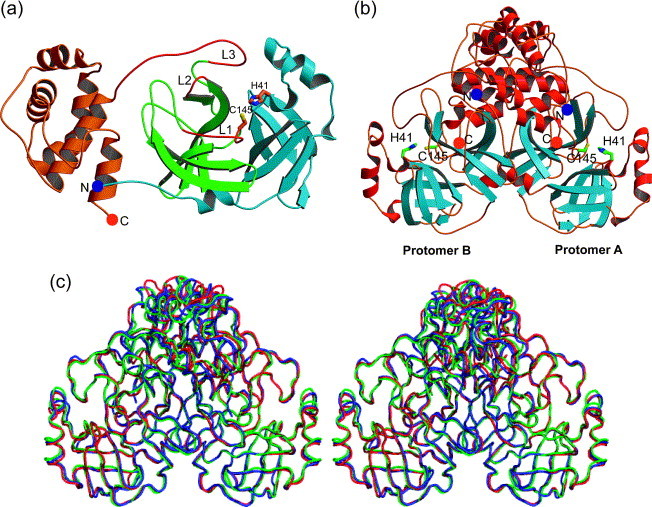
Structure of the (a) monomer and (b) dimer of SARS-CoV Mpro. (a) Domains I (light blue) and II (green) each contain a six-stranded β-barrel and domain III (orange) is composed mainly of α-helices. The amino and the carboxy terminus are marked by a blue and an orange sphere, respectively. The flexible loops L1, L2, and L3 (red) comprise residues 138–145 (the oxyanion-binding loop), 165–172, and 185–200, respectively. (b) α-Helices are red and β-strands are light blue. The amino and the carboxy termini are marked by blue and orange spheres, respectively. Dimerization is mainly due to interactions between the helical domains III of each monomer (top). (c) Superimposition (in stereo) of the Cα backbone as determined in three different crystal forms. Blue, monoclinic form; red, tetragonal form; green, orthorhombic form. (a) and (b) were prepared by MOLSCRIPT,40 (c) was prepared by PyMOL.41
