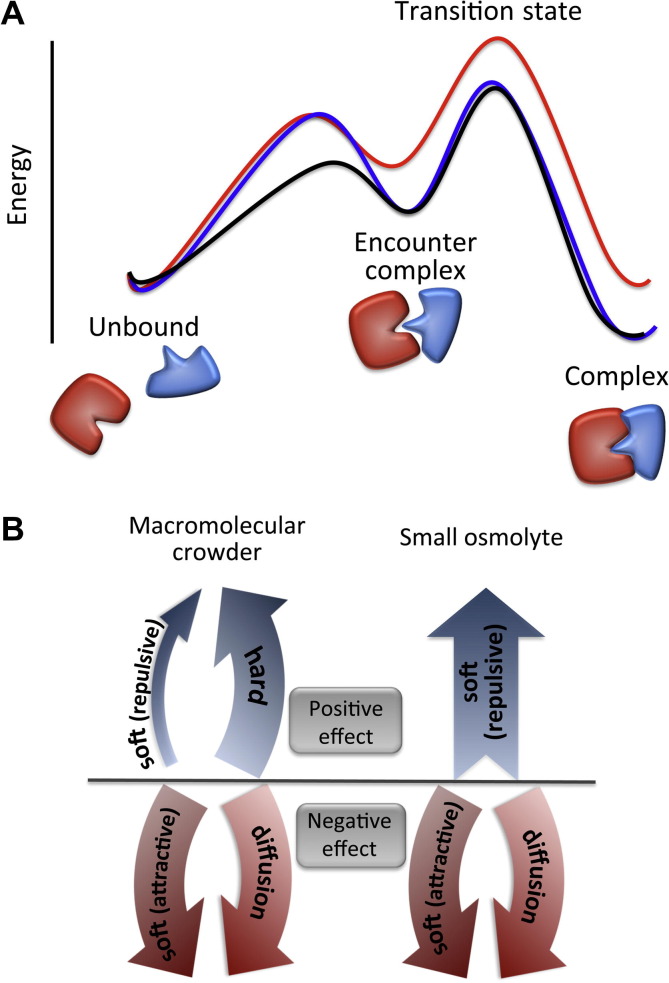
Fig. 2.
Energy diagram demonstrating postulated effect of excluded volume and viscosity on the association pathway. In the presence of macromolecular crowders (blue), formation of the encounter complex would be slower compared with dilute solution (black) due to slow protein diffusion. Dissociation of encounter complex back to unbound proteins would be slower due to the depletion force, and thus encounter complex stability is similar to that in dilute solution. When the solution becomes viscose without being volume-occupied (red, as in the case of solutions containing small osmolytes), no stabilizing force counter-balance the reduced diffusion of proteins. Note that this diagram refers only to relative energy levels between states along the same association pathway, not to relative energy levels between the same states at different association pathways. The effect of hard (steric) interactions, soft (electrostatic, hydrophobic and van der Waals) interactions and diffusion on protein association rate is shown in (B). Attractive soft interactions between crowders and proteins may reduce the association rate, while repulsive soft interaction (e.g. electrostatic repulsion) may increase the association rate.