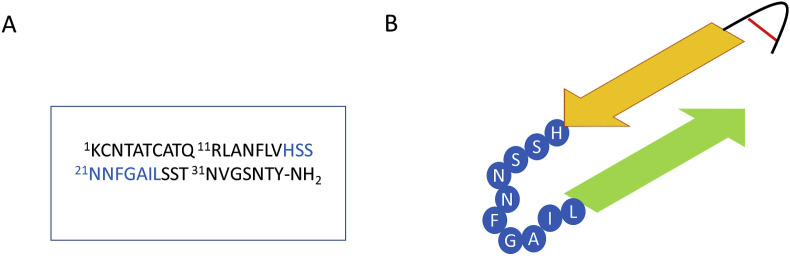
Fig. 3.
A) The sequence of human amylin. B) Final form adopted by human amylin monomers in amyloid fibrils. The residues 18–27 of hAM are highlighted in blue in both A) and B) because of the conflicting observations that on the one hand studies show that this is the minimum sequence of hAM capable of forming amyloid fibrils and yet on the other there are indications that in the final amyloid configuration this region has no β-sheet character, rather, it seems to exist as an unstructured loop. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)