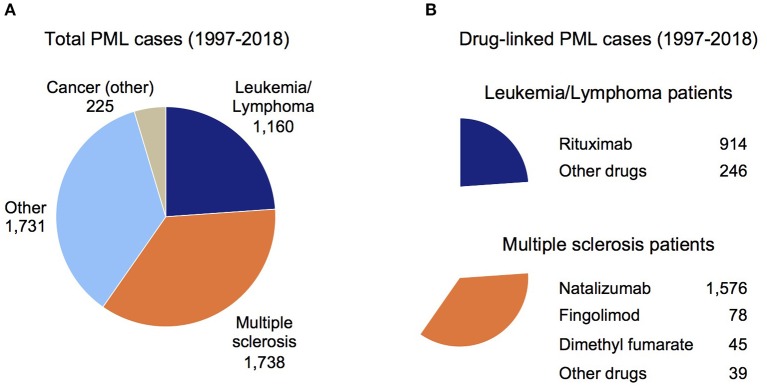
Figure 1.
Overview of PML cases in the FDA Adverse Event Reporting System (FAERS). (A) Total PML cases reported in the FAERS database sub-grouped by the patient's underlying disease (see Methods). (B) Total drug-linked PML cases for the Leukemia/Lymphoma patients (top chart) and MS patients (bottom chart). The largest number of drug-linked PML cases in these disease groups were found for rituximab and natalizumab (Other drugs subgroup contains drugs linked to a small number of PML cases).