Figure 4. Mean change in log10 HCV RNA with 90% confidence intervals after administration of single oral doses of BMS-790052 to HCV-infected patients.
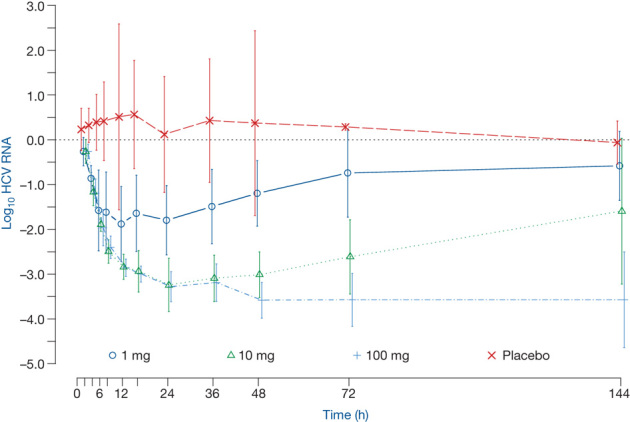
In a double-blind, placebo-controlled, sequential, single ascending-dose study, six subjects were randomized within each dose panel (1, 10, 100 mg) to drug or placebo in a ratio of 5:1. BMS-790052 or placebo was administered in the fasted state. Owing to a dosing error, all six subjects received BMS-790052 in the 1 mg panel. One subject in the 10 mg panel withdrew from the study 8 h after administration of the study drug for non-drug-related reasons; HCV RNA data from the subject are included up until the subject withdrew.
