Fig. 3. SUMOylation enhances DUSP6 stability by repressing its ubiquitination.
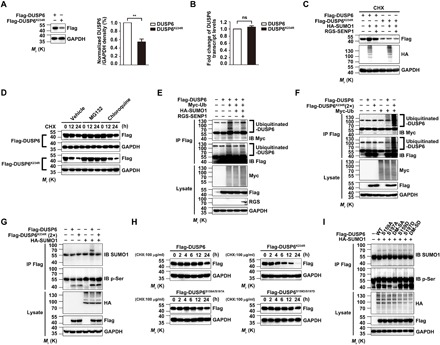
(A and B) The SUMOylation-deficient DUSP6K234R mutant exhibits reduced protein, but not RNA, expression as compared to the wild type. Lysates from HeLa cells transiently transfected with Flag-DUSP6 or Flag-DUSP6K234R were subjected to IB with the anti-Flag antibody to determine protein expression (A) and real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) to measure the DUSP6 transcript levels (B). Results normalized to wild-type DUSP6 were summarized for three independent experiments and shown as means ± SEM. **P < 0.01, by two-tailed Student’s t test. (C) SUMOylation increases the stability of DUSP6 but not the DUSP6K234R mutant. Lysates from HeLa cells transiently transfected with Flag-DUSP6, Flag-DUSP6K234R, HA-SUMO1, or RGS-SENP1 at various combinations as indicated and treated with CHX (100 μg/ml) for 12 hours beginning at 24 hours after transfection to prevent de novo protein synthesis were analyzed by IB with anti-Flag, anti-HA, and anti-GAPDH. Note that, in the presence of CHX, the increase in DUSP6 level due to HA-SUMO1 overexpression indicates decreased degradation, which was reversed by RGS-SENP1; however, the level of DUSP6K234R is unaffected by HA-SUMO1 and RGS-SENP1. (D) DUSP6K234R exhibits faster degradation via the proteasome pathway than wild-type DUSP6. HeLa cells transiently transfected with Flag-DUSP6 or DUSP6K234R were treated with CHX (100 μg/ml) for 0 to 24 hours as indicated beginning at 24 hours after transfection. Dimethyl sulfoxide (vehicle control), proteasome inhibitor, MG132 (20 μM), or lysosome inhibitor, chloroquine (100 μM), was added 1 hour before CHX. Cell lysates were subjected to IB using anti-Flag and anti-GAPDH antibodies. Note the faster decrease in DUSP6K234R than DUSP6 and the blockade by MG132 but not chloroquine. (E) SUMO1 modification of DUSP6 represses its ubiquitination. Lysates from HeLa cells transiently transfected with Flag-DUSP6, HA-SUMO1, Myc-ubiquitin (Myc-Ub), or RGS-SENP1 at various combinations as indicated were subjected to IP with anti-Flag antibody under denaturing conditions, which was followed by IB with anti-Myc and anti-Flag antibodies. The original lysates were also analyzed by IB for inputs of Myc-Ub, Flag-DUSP6, and RGS-SENP1, with GAPDH as the loading control. (F) The SUMOylation-deficient DUSP6K234R mutant has enhanced ubiquitination as compared to wild-type DUSP6. Similar to (E) but the cells were transfected with Flag-DUSP6, Flag-DUSP6K234R (2×), or Myc-Ub at various combinations as indicated. Note the stronger Myc-Ub labeling samples transfected with Flag-DUSP6K234R than Flag-DUSP6. (G) DUSP6 SUMOylation has no impact on its phosphorylation. Lysates from HeLa cells transiently transfected with Flag-DUSP6, Flag-DUSP6K234R (2×), or HA-SUMO1 at various combinations as indicated were subjected to IP with the anti-Flag antibody, which was followed by IB with anti-SUMO1 and anti–p-Ser antibodies. The original lysates were also analyzed by IB for the inputs of HA-SUMO1 and Flag-DUSP6, with GAPDH as the loading control. Note the similar levels of p-Ser labels between DUSP6 and DUSP6K234R and the lack of effect of HA-SUMO1. (H) DUSP6 SUMOylation, but not phosphorylation, regulates its degradation. Lysates from HeLa cells transiently transfected with Flag-DUSP6, Flag-DUSP6K234R, or phosphorylation-defective (S159A/S197A, DM-SA) or phosphorylation-mimetic (S159D/S197D, DM-SD) DUSP6 mutant and treated with CHX (100 μg/ml) for 0 to 24 hours as indicated beginning at 24 hours after transfection were subjected to IB with anti-Flag and anti-GAPDH antibodies. Note that the phosphorylation-defective and phosphorylation-mimetic DUSP6 mutants exhibited similar stability to the wild-type protein. (I) DUSP6 phosphorylation has no impact on its SUMOylation. Lysates from HeLa cells transiently cotransfected with HA-SUMO1 and Flag-DUSP6, or one of its phosphorylation-defective or phosphorylation-mimetic mutants as indicated for 24 hours were subjected to IP and IB similarly as in (G). Note the similar levels of SUMOylation for DUSP6 and all its mutants. In (C) to (I), blots are representatives of at least three independent experiments. Note that 2× amount of DUSP6K234R plasmid was used for transfection in some experiments to ensure comparable protein expression with the wild type.
