Fig. 2. Human light chain T99wt and the mutant, T99-Pro95(−).
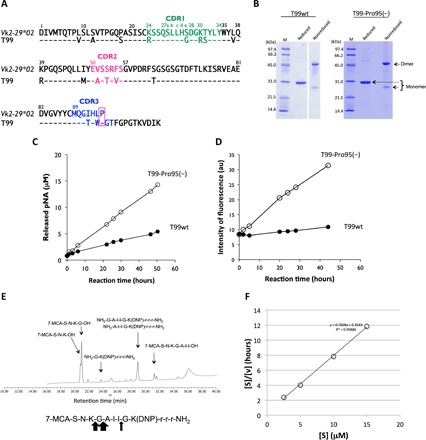
(A) Comparison of germline (Vk2-29*02) and T99wt. The sequence of the Vκ region (amino acids 1 to 95) of T99wt is compared with that of a germline Vk2-29*02. Amino acids in the sequence of T99wt are different at many positions from those of the germ line, indicating many somatic mutations. (B) SDS-PAGE analysis of T99wt and T99-Pro95(−) light chain. Bands were visualized by CBB staining. Similar bands to those observed in S35 and S38 were seen under both the reduced and nonreduced conditions. The bands at about 46 and 26 kDa correspond to the dimer and monomer, respectively, under the nonreduced condition. The clear band at about 30 kDa corresponds to the monomer under the reduced condition. (C) Time course of the cleavage reaction for Arg-pNA. T99-Pro95(−) (10 μM: open circle) clearly exhibited much higher catalytic activity than T99wt (10 μM: closed circle) to cleave the Arg-pNA substrate. In this case, the deletion of Pro95 in complementarity-determining region 3 (CDR-3) enhanced peptidase activity. The reaction was carried out in triplicate at 37°C. (D) Time course of the cleavage reaction for FRET-Aβ peptide. FRET-Aβ:25 μM. T99wt: 5 μM (closed circle). T99-Pro95(−): 5 μM (open circle). The T99-Pro95(−) light chain exhibited the catalytic activity to cleave FRET-Aβ, while T99wt did not. Deletion of Pro95 contributed to acquisition of the catalytic function. au, arbitrary units. (E) HPLC analysis of the reaction products. Cleaved bonds of FRET-Aβ by T99-Pro95(−) were investigated. Fragments of 7-MCA-S-N-K-G-OH (21 min) and NH2-A-I-I-G-K(DNP)-r-r-r-NH2 (29 min) show that the Gly-Ala peptide bond was cleaved. Fragments of 7-MCA-S-N-K-OH (20.6 min) and NH2-G-A-I-I-G-K(DNP)-r-r-r-NH2 (29 min) show that the Lys-Gly was cleaved. Ile-Gly peptide bond was slightly cleaved. The Gly-Ala peptide bond was mostly cleaved. (F) Kinetic analysis by T99-Pro95(−). T99-Pro95(−); 5 μM, FRET-Aβ; 5 ~ 60 μM [S]; concentration of FRET-Aβ; [V]; initial rate. The Hanes-Woolf plot demonstrates that the cleavage reaction by T99-Pro95(−) light chain fits the Michaelis-Menten kinetics equation, indicating that the reactions are enzymatic.
