Fig. 4. Molecular modeling of light chains.
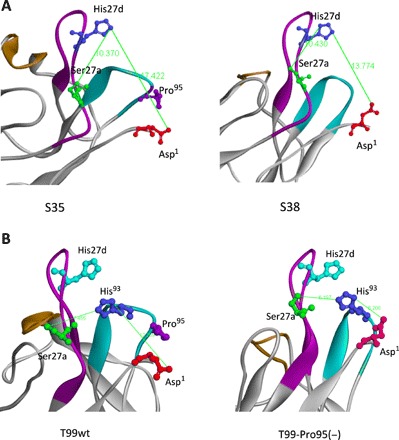
(A) S35 and S38 light chains. In the case of S38, the amino acid residues of Asp1, Ser27a, and His27d (or His93) are considered to form a catalytic triad-like structure, although the distances are slightly long. The distance between Ser27a(O) and His27d (N) is 10.37 Å in S35 and 10.43 Å in S38. The distance between His27d(N) and Asp1(O) is 17.42 Å in S35 and 13.77 Å in S38. The S38 distance is shorter than the S35 distance by 3.65 Å, suggesting that Asp1 can approach His27d. (B) T99wt and T99-Pro95(−) light chains. In this case, a catalytic triad composed of Asp1, Ser27a, and His93 is preferable. The distance between Ser27a(O) and His93(N) is 7.66 Å in T99wt and 6.20 Å in T99-Pro95(−). The distance between His93(N) and Asp1(O) changed from 12.26 Å in T99wt to 6.208 Å in T99-Pro95(−), and the residues face each other. This is a crucial change from which the catalytic triad can be derived.
