Abstract
Thirdhand smoke (THS), the residual tobacco smoke remaining in the environment after tobacco has been smoked, represents a hidden and underestimated public health hazard. Evidence supports its widespread presence in indoor environments. Exposure to secondhand smoke (SHS), a precursor of THS, has been well documented as a risk factor for human cancers, especially lung cancer. However, the concept of THS as a distinct entity that poses health risks for small children has developed only recently and the associations of THS with cancer risk and other chronic diseases are poorly understood due to limited numbers of studies to date. In this perspective, we mainly summarize all published studies on the genotoxicity and carcinogenic potential of THS exposure. These studies begin to fill the knowledge gap in our understanding of cancer risk of THS. Accumulating data from existing and future studies will help reduce the tobacco-related cancer incidence through changes in lifestyle and tobacco control policies.
Keywords: Tobacco, Thirdhand smoke, Tobacco-specific nitrosamines, Genotoxicity, Carcinogenesis
Introduction
Thirdhand smoke (THS) is a newly recognized tobacco smoke hazard and has become a public concern in recent years because of its wide presence in indoor environment and significant adverse biological and health effects.1, 2 By definition, THS is the contamination of surfaces in contact with compounds emitted in secondhand smoke (SHS), the products generated by chemical transformations of these components, and the off-gassing of volatile components into the air.1, 2 The “four Rs” can define the concept of THS: tobacco chemicals (some toxic) that Remain, React, Re-emit and/or are Re-suspended long after active smoking ends. Compared to SHS, the timescale for exposure to THS is typically much longer, e.g., weeks and months.3, 4 Moreover, THS is difficult to remove from the polluted environment. SHS and THS often co-exist in the environment and together are referred to as “passive smoke”. Small children are believed to be particularly vulnerable to THS exposure because of their age-related metabolic and behavior characteristics.1, 2
Interestingly, the first studies on THS toxicity go back to the 1980s and were done by tobacco industry. The secondary analysis of animal experiments carried out by the Philip Morris Tobacco Company revealed that sidestream smoke, a form of SHS, is two to four times more toxic than mainstream smoke per gram of tobacco and that sidestream condensate is approximately two to six times more tumourigenic per gram of tobacco compared to mainstream condensate by dermal application in rats.5 Moreover, it was found that “aged” sidestream smoke is 6–12 times more toxic than fresh sidestream smoke.6 This increase in toxicity over time is particularly interesting and attributed to what could occur as SHS evolves into THS. In fact, one of the major advances in recent years was the discovery of novel chemical constituents generated de novo in THS. For example, indoor chemistry studies have elegantly revealed that surface-bound nicotine, a major constituent of THS, reacts with nitrous acid (HONO) to form carcinogenic tobacco-specific nitrosamines (TSNAs),7 and with ozone (O3) to yield toxic aldehydes.8 HONO is an air pollutant produced by improperly vented indoor combustion sources such as gas stoves and also in vehicle exhaust. As for indoor ozone, the source is mainly from outdoor ozone penetration. In addition, a common source of indoor ozone is air generators or purifiers used to remove intense tobacco odors. Therefore, the “aging process” of SHS, i.e., the formation of THS, has become a focus of research interest since it may reveal a hidden environmental risk factor especially for children and non-smokers.9
Studies over the last decade have substantially increased our understanding of the chemistry, exposure assessment, biomarkers and potential biological/health effect of THS,1, 2, 10, 11 after the term “THS” started to appear in public.12, 13, 14 Although compelling evidence now shows that THS and some of its constituents such as TSNAs can cause significant cellular and tissue changes at environmentally relevant doses, its effect on cancer risk in exposed populations is still poorly understood. It has been well known that cigarette smoking is directly associated with an increased cancer incidence in multiple organs, of which, the lung is particularly vulnerable as approximately 90% of its cancer cases are associated with cigarette smoking.15 Based on the International Agency for Research on Cancer (IARC), there is also sufficient evidence that SHS causes lung cancer.15
Through reviewing the published studies, this perspective summarizes the current evidence supporting the mutagenic and carcinogenic potential of THS, the underlying mechanisms and its impact on health. Other comprehensive reviews covering various aspects of THS research have been published previously.1, 2, 16, 17, 18
THS contains many mutagens and carcinogens
A large number of toxic compounds, including those produced de novo, have been identified from THS in laboratory systems and in field studies.1, 2 It is well known that tobacco smoke is a rich source of mutagens and carcinogens, including polycyclic aromatic hydrocarbons (PAHs), hydrocarbons, nitrosamines, aromatic amines, aldehydes, phenolic and nitro compounds, as well as other inorganic and organic compounds.19, 20, 21 Mainstream smoke contains over 60 carcinogens classified by IARC.15 SHS also contains many of the same species as those in mainstream smoke, and most of the carcinogens in mainstream smoke are also present in SHS.22
THS chemical composition is affected by air−surface partitioning and by chemical transformations. Chemical analyses by various laboratories have revealed many classes of toxic constituents in THS from both laboratory and real life environments. Researchers have shown that indoor surfaces adsorb semi-volatile organic compounds (SVOCs) from SHS, such as nicotine, cotinine, 3-ethenylpyridine, PAHs, N-nitrosamines, aromatic amines (e.g., naphthalene), which are then slowly released into the air,23, 24, 25, 26, 27, 28, 29, 30 increasing exposure risk. Sleiman et al31 studied 58 volatile organic compounds (VOCs) during the aging process of SHS and observed that many of them such as acrolein, furan, acrylonitrile, and 1,3-butadiene persisted and were present at higher concentrations in the gas phase with time. Of the chemical compounds identified in THS, human carcinogens include PAHs, nitrosamines, 1,3-butadiene and benzene; possible human carcinogens are naphthalene and furans (based on IARC and National Toxicology Program).32
Studies have also been focused on new chemical compounds generated in THS de novo. In 2010, Sleiman et al7 reported that in the laboratory, nicotine reacts with HONO to form several TSNAs including 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), 1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal (NNA), and N-nitrosonornicotine (NNN). NNK has also been identified in real life places such as smokers' homes.33 NNK and NNN are classified human carcinogens and there is some evidence for the mutagenicity of NNA.34 The extent of surface loading of these compounds was estimated to be large enough to warrant investigation of health effects of THS exposure.7 Another example of chemical transformation is that surface nicotine reacts with ozone in the air to form fine particles as well as carcinogenic compounds such as formaldehyde and N-methylformamide.8 Together, these studies provide evidence for the unsure toxicological and dynamic nature of THS formation and composition. The critical question of what toxicants are present in THS and at what quantities remains under investigation.
THS exposure induces various cellular responses
THS exposure alters transcriptional profile and activates p53 signaling
To elucidate the molecular and cellular mechanisms underlying THS-induced cancer, we exposed human cells to THS for 12 consecutive days followed by RNA-sequencing.35 The data revealed 1385 differentially expressed genes and a significant overlap with a published non-tumor human lung tissue smoking signature,36 suggesting that at least part of the in vitro transcriptional response to THS is similar to transcriptional changes in active smokers. We next computationally mapped these genes to biological functions, pathways and upstream transcriptional regulators and found that THS modulated gene expression profile enriched for certain pathways, most noticeably the activation of the p53 signaling pathway. It is well established that the p53 gene, as a critical tumor suppressor, responds to a variety of cellular stresses such as DNA damage, activates DNA repair machinery and plays a key role in cancer development.37
THS exposure increases cellular growth transformation in vitro
To assess the effect of THS exposure on cancer development, we employed the soft agar transformation assay to test anchorage independent growth, a key indicator of neoplastic transformation. We found that THS extracts-treated lung cancer cell lines H460 and H510 exhibited increased cell proliferation compared to control cells.35 Furthermore, THS chronically treated H460 and H510 cells exhibited significant increases in the number of colonies compared to controls, indicating that THS exposure causes cell transformation in human cells.35
THS exposure increases lung cancer incidence in A/J mice
A fundamental question for both the research community and the general public is whether THS increases cancer risk. Although Martins-Green et al38 observed adverse effects on multiple organ systems when C57BL/6 mice were exposed for 6 months starting at 3 weeks of age, there was no increased cancer incidence.
We recently used A/J mice, which are susceptible to lung cancer development, to investigate the carcinogenic effect of THS.35 Mice were exposed to THS for 3 weeks from the 4–7 weeks of age, and monitored for tumor development until 47 weeks of age. A significant increase in the proportion of mice with lung adenocarcinoma was identified in the THS-exposed cohort compared to controls.35 This study provided the first evidence that exposure to THS could increase cancer risk in an in vivo mammalian system.
THS exposure and increased cancer risk in children
It was reported that the ratio of urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL)/cotinine was higher in children compared to adults, providing evidence that children are particularly vulnerable to environmental tobacco smoke exposure.39 NNAL is a metabolite of NNK, and is currently utilized as a biomarker for SHS or THS exposure and a risk indicator for pulmonary carcinogenesis.2, 40, 41, 42 Another study provided evidence for uptake of nicotine, NNK, and acrolein in children living in homes of hookah smokers.43 Ramírez et al44 estimated the potential cancer risk through ingestion and dermal exposure to carcinogenic N-nitrosamines and TSNAs measured in house dust samples by applying the most recent official toxicological information and concluded that the calculated cancer risks through exposure to TSNAs increase at an early life stage, that is, 1–6 years old.
THS exposure causes DNA damage
Cancer arises from both genetic and epigenetic alterations of DNA contributing to mutations and/or changes in gene expression. It is well known that the environmental pollutants that play key roles in the etiology of human cancer include chemical mutagens and carcinogens in tobacco smoke.19, 20 One of the main research tasks is to identify those constituents in THS that are toxic and/or carcinogenic. Mechanistically, THS could induce DNA damage as well as epigenetic changes, which result in mutations and changes in gene expression associated with cancer development. To confirm the carcinogenic potential of THS, both animal studies and human epidemiological investigations are warranted.
THS induces DNA strand breaks and oxidative base damage
To assess the genotoxicity of THS in vitro, in 2013, we first reported that exposure of human hepatocellular carcinoma (HepG2) cells for 24 hours to either acute or chronic laboratory prepared THS samples resulted in a significant increase in DNA strand breaks using the Comet assay.45 Using the same assay, Bahl et al11 also reported the formation of DNA strand breaks in mouse neural stem cells and human dermal fibroblasts after THS exposure. The concentrations of nicotine, nicotine related alkaloids, and TSNAs (NNK, NNN and NNA) in fresh and aged extracts were analyzed, and showed that the concentration of TSNAs increased with time.11, 45 In these experiments, the levels of THS exposure were comparable to exposure levels in homes and other public places. Later, using a double-strand break (DSB) marker γ-H2AX, we confirmed the formation of DSBs in exposed human bronchial epithelial cells (BEAS-2B) and two lung cancer cell lines.35 DSB is the most lethal type of DNA damage, as both strands of the DNA duplex are compromised.46 It should be noted that NNA also induces DNA strand breaks in human HepG2 using the Comet assay with NNK exposure as a positive control.45 NNA caused DNA strand breaks at non-cytotoxic doses, confirming the genotoxic potential of NNA.
We also found significantly higher levels of oxidative DNA damage in hypoxanthine phosphoribosyltransferase 1 (HPRT1) and DNA polymerase β (POLB) genes of human BEAS-2B cells exposed to THS, using the long amplicon (LA)-quantitative polymerase chain reaction assay.45 This assay can detect DNA lesions such as those caused by oxidative damage with a high sensitivity.47, 48, 49 THS also causes oxidative damage in these two genes in lung fibroblasts isolated from Sprague–Dawley rat pups using the same assay, together with increased cell apoptosis, and differentiation to a myogenic phenotype in such cells, suggesting that THS exposure could result in chronic lung damage in THS exposed infants.47 Furthermore, we found increased levels of oxidative DNA damage in the same genes in mouse skin wounds exposed to THS.48 This finding was in agreement with increased levels of the oxidative DNA damage biomarker 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) in the same tissues, as found by Dr. Martins-Green's laboratory.48 These results strongly suggest that exposure to THS results in oxidative stress in cells or organisms, which could lead to disease-causing mutations, and increased cancer risk.50 We previously found in metabolomics studies that glutathione metabolism was significantly activated in mouse male reproductive GC-2 cells after THS exposure.51 A THS-induced increase of oxidative stress and oxidation of mitochondrial proteins was also observed by Bahl et al.52 The relationship between THS exposure, oxidative stress, reactive oxygen species (ROS) production and antioxidant response mediated by activated glutathione metabolism awaits further investigation.
NNA induces the formation of DNA adducts
It is well accepted that the formation of DNA adducts plays a central role in smoking-induced mutagenesis and carcinogenesis. NNK is a potent lung carcinogen and induces bulky DNA adducts in lung tissue of experimental animals and smokers.53, 54, 55 NNA was identified as the major TSNA product when nicotine reacts with HONO, which is absent in freshly emitted tobacco smoke.7 Therefore, it would be important to assess its intake and biological properties. NNA is a reactive aldehyde with a mutagenic activity similar to that of NNN, a human carcinogen,32 but its tumorigenic activity in animals is not conclusive.19, 56 With the use of liquid chromatography (LC)-electrospray ionization tandem mass spectrometry (ESI-MS/MS) and 2D nuclear magnetic resonance (NMR), our group first identified DNA adducts from reaction of NNA with 2′-deoxynucleosides, including a novel adduct, 1,N2-NNA-dG, along with N1-, O6-methyl-dG and 8-oxodG, from in vitro NNA-dG reactions.57 On C-18 high performance liquid chromatography (HPLC), 1,N2-NNA-dG appeared as the major adduct and ESI-MS/MS showed a product of m/z 455.17 for (M+1)+, which seems to result from the condensation of NNA and dG with the elimination of H2O and two H molecules.57 Given that NNA is highly selective for THS, these data also suggest that 1,N2-NNA-dG is a promising specific biomarker for THS exposure, in addition to its role in carcinogenesis.
Summary and perspective
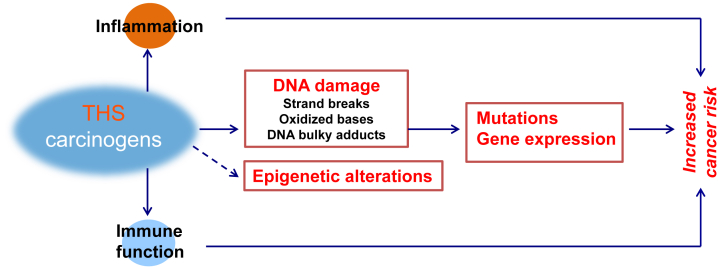
THS pollution is a worldwide issue, especially in countries with a high prevalence of smoking.1, 2, 58 As described above, recent progress on the effects of THS on genotoxicity, early life development and cancer induction in cellular and animal models indicates that THS is genotoxic and carcinogenic at environmentally relevant doses. The underlying mechanisms of THS-induced disease should be explored using an integrative systems biology approach combining data at the cellular level with holistic data obtained investigating organism level responses including for example THS-effects on the immune system35, 59 (Fig. 1). Systems biology, unlike traditional approaches to the analysis of disease that focus on single genes or proteins in isolation, attempts to integrate the complex interaction of many kinds of genetic and biological information — genomic DNA sequence, messenger RNA (mRNA) and protein expression, and link these to disease phenotypes. A systems biology approach to THS-induced disease involves taking a comprehensive global view of the various sub-phenotypes that contribute to cancer, and asking how these are associated with the normal genetic and gene expression architecture of the host, as well as the changes in this architecture that accompany disease development and progression. However, direct human studies are likely to be fraught with difficulties since the majority of humans are exposed to mixed secondhand and thirdhand smoke. Moreover, diverse confounding factors in humans, such as other environmental pollutants, likely limit our ability to draw definitive conclusions about the health impact of THS. In contrast, model systems including mouse models offer the possibility of controlling both the environmental exposures and the effects of genetic background, with the result that many of the confounding factors in human cancer studies can be eliminated.
Fig. 1.
Scheme for proposed mechanistic framework showing how thirdhand smoke may induce cancer.
One important aspect of a systems biology approach is to investigate the interplay between genetic susceptibility and THS carcinogenicity, which still remains unknown.60 Suitable population-based animal model systems such as the Collaborative Cross (CC) mouse resource or the Diversity Outbred (DO) mouse population should be used to investigate linkage of genetic variants to exposure-response outcomes. These mouse populations contain a level of genetic and phenotypic diversity on par with the human population and harbor sufficient genetic diversity to identify genetic variants associated with THS exposure-induced health effects including tumor development. Previous studies have shown the human relevance of data obtained using these population-based models by extrapolating to human disease.61 Moreover, these studies will help discover the molecular mechanisms underlying THS-induced disease and identify biomarkers of THS exposure.
More recently, the power of artificial intelligence and machine learning has been brought to bear on systems biology approaches in medicine and biomedical research. One example is the use of a multi-scale convolutional sparse coding framework (MSCSC), which improves the current state-of-the-art by enforcing the unsupervised joint learning of scale-specific knowledge simultaneously across different scales.62 This approach allows straightforward multi-modality extension in a novel deep architecture and enables efficient and effective integration of information from multiple modalities (e.g. different genetics and OMICS analyses) to ultimately capture complex multi-modality signatures that contribute to our understanding of THS-induced disease risk.
In addition to model system studies, the impact of long-term exposure to THS on cancer risk should also be further investigated in human cohort studies focused on those at the greatest risk including young children. In parallel, focus should be put on developing strategies for remediation and prevention limiting children's exposure.63, 64, 65 Combing these studies will ultimately contribute significantly to better prevention of THS exposure-induced health effects in humans and provide further scientific rationale for policy decisions.
Conflicts of interest
None.
Acknowledgments
This work was supported by the University of California Tobacco-Related Disease Research Program (TRDRP) research project grants 24RT-0038 (BH and JHM) and 28PT-0076 (BH, JHM and AMS).
Edited by Pei-Fang Wei
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Jacob P., 3rd, Benowitz N.L., Destaillats H. Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem Res Toxicol. 2017;30:270–294. doi: 10.1021/acs.chemrestox.6b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matt G.E., Quintana P.J., Destaillats H. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011;119:1218–1226. doi: 10.1289/ehp.1103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matt G.E., Quintana P.J., Zakarian J.M. When smokers move out and non-smokers move in: Residential thirdhand smoke pollution and exposure. Tob Control. 2011;20:e1. doi: 10.1136/tc.2010.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matt G.E., Quintana P., Zakarian J.M. When smokers quit: Exposure to nicotine and carcinogens persists from thirdhand smoke pollution. Tob Control. 2016;26:548–556. doi: 10.1136/tobaccocontrol-2016-053119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schick S., Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob Control. 2005;14:396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schick S.F., Glantz S.A. Sidestream cigarette smoke toxicity increases with aging and exposure duration. Tob Control. 2006;15:424–429. doi: 10.1136/tc.2006.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleiman M., Gundel L.A., Pankow J.F., Jacob P., 3rd, Singer B.C., Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Destaillats H., Singer B.C., Lee S.K., Gundel L.A. Effect of ozone on nicotine desorption from model surfaces: Evidence for heterogeneous chemistry. Environ Sci Technol. 2006;40:1799–1805. doi: 10.1021/es050914r. [DOI] [PubMed] [Google Scholar]
- 9.Hang B., Chenna A., Gundel L.A. Smoke: The hidden risks. Chem Ind. 2014;78:36. [Google Scholar]
- 10.Hang B., Wang P., Zhao Y. Adverse health effects of thirdhand smoke: From cell to animal models. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18050932. pii: E932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahl V., Shim H.J., Jacob P., 3rd, Dias K., Schick S.F., Talbot P. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol In Vitro. 2016;32:220–231. doi: 10.1016/j.tiv.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo L. Babies may absorb smoke residue in home. USA Today. August 6, 2006 http://tinyurl.com/zhoke [Google Scholar]
- 13.Winickoff J.P., Friebely J., Tanski S.E. Beliefs about the health effects of "thirdhand" smoke and home smoking bans. Pediatrics. 2009;123:e74–e79. doi: 10.1542/peds.2008-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabin R.C. A new cigarette hazard: ‘Third-Hand Smoke’. New York Times. January 2, 2009 http://tinyurl.com/9g9vrk [Google Scholar]
- 15.International Agency for Research on Cancer . 2004. Tobacco smoke and involuntary smoking; pp. 1189–1413. (IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans. No. 83. 121–844. Lyon, France). [Google Scholar]
- 16.Burton A. Does the smoke ever really clear? Thirdhand smoke exposure raises new concerns. Environ Health Perspect. 2011;119:A70–A74. doi: 10.1289/ehp.119-a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díez-Izquierdo A., Cassanello-Peñarroya P., Lidón-Moyano C., Matilla-Santander N., Balaguer A., Martínez-Sánchez J.M. Update on thirdhand smoke: A comprehensive systematic review. Environ Res. 2018;167:341–371. doi: 10.1016/j.envres.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Torres S., Merino C., Paton B., Correig X., Ramírez N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: Recent advances and future perspectives. Int J Environ Res Public Health. 2018;15:2693. doi: 10.3390/ijerph15122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hang B. Formation and repair of tobacco carcinogen-derived bulky DNA adducts. J Nucleic Acids. 2010;2010:709521. doi: 10.4061/2010/709521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hecht S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 21.Smith C.J., Perfetti T.A., Garg R., Hansch C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41:807–817. doi: 10.1016/s0278-6915(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 22.California Environmental Protection Agency Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. https://ww3.arb.ca.gov/toxics/id/summary/etspt_a.pdf
- 23.Van Loy M.D., Riley W.J., Daisey J.M., Nazaroff W.W. Dynamic behavior of semivolatile organic compounds in indoor air. 2. Nicotine and phenanthrene with carpet and wallboard. Environ Sci Technol. 2001;35:560–567. doi: 10.1021/es001372a. [DOI] [PubMed] [Google Scholar]
- 24.Singer B.C., Hodgson A.T., Nazaroff W.W. Gas-phase organics in environmental tobacco smoke: 2. Exposure-relevant emission factors and indirect exposures from habitual smoking. Atmos Environ. 2003;37:5551–5561. [Google Scholar]
- 25.Hoh E., Hunt R.N., Quintana P.J. Environmental tobacco smoke as a source of polycyclic aromatic hydrocarbons in settled household dust. Environ Sci Technol. 2012;46:4174–4183. doi: 10.1021/es300267g. [DOI] [PubMed] [Google Scholar]
- 26.Fleming T., Anderson C., Amin S., Ashley J. Third-hand tobacco smoke: Significant vector for PAH exposure or non-issue. Integr Environ Assess Manag. 2012;8:763–764. doi: 10.1002/ieam.1337. [DOI] [PubMed] [Google Scholar]
- 27.Schick S.F., Farraro K.F., Perrino C. Thirdhand cigarette smoke in an experimental chamber: Evidence of surface deposition of nicotine, nitrosamines and polycyclic aromatic hydrocarbons and de novo formation of NNK. Tob Control. 2014;23:152–159. doi: 10.1136/tobaccocontrol-2012-050915. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez N., Vallecillos L., Lewis A.C., Borrull F., Marcé R.M., Hamilton J.F. Comparative study of comprehensive gas chromatography-nitrogen chemiluminescence detection and gas chromatography-ion trap-tandem mass spectrometry for determining nicotine and carcinogen organic nitrogen compounds in thirdhand tobacco smoke. J Chromatogr A. 2015;1426:191–200. doi: 10.1016/j.chroma.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Whitlatch A., Schick S. Thirdhand Smoke at Philip Morris. Nicotine Tob Res. 2018 doi: 10.1093/ntr/nty153. [DOI] [PubMed] [Google Scholar]
- 30.Bahl V., Weng N.J., Schick S.F. Cytotoxicity of thirdhand smoke and identification of acrolein as a volatile thirdhand smoke chemical that inhibits cell proliferation. Toxicol Sci. 2016;150:234–246. doi: 10.1093/toxsci/kfv327. [DOI] [PubMed] [Google Scholar]
- 31.Sleiman M., Logue J.M., Luo W., Pankow J.F., Gundel L.A., Destaillats H. Inhalable constituents of thirdhand tobacco smoke: Chemical characterization and health impact considerations. Environ Sci Technol. 2014;48:13093–13101. doi: 10.1021/es5036333. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services 14th Report on Carcinogens (RoC) https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html
- 33.Thomas J.L., Hecht S.S., Luo X., Ming X., Ahluwalia J.S., Carmella S.G. Thirdhand tobacco smoke: A tobacco-specific lung carcinogen on surfaces in smokers' homes. Nicotine Tob Res. 2014;16:26–32. doi: 10.1093/ntr/ntt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespi C.L., Penman B.W., Gelboin H.V., Gonzalez F.J. A tobacco smoke-derived nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, is activated by multiple human cytochrome P450s including the polymorphic human cytochrome P4502D6. Carcinogenesis. 1991;12:1197–1201. doi: 10.1093/carcin/12.7.1197. [DOI] [PubMed] [Google Scholar]
- 35.Hang B., Wang Y., Huang Y. Short-term early exposure to thirdhand cigarette smoke increases lung cancer incidence in mice. Clin Sci (Lond) 2018;132:475–488. doi: 10.1042/CS20171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossé Y., Postma D.S., Sin D.D. Molecular signature of smoking in human lung tissues. Cancer Res. 2012;72:3753–3763. doi: 10.1158/0008-5472.CAN-12-1160. [DOI] [PubMed] [Google Scholar]
- 37.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins-Green M., Adhami N., Frankos M. Cigarette smoke toxins deposited on surfaces: Implications for human health. PLoS One. 2014;9:e86391. doi: 10.1371/journal.pone.0086391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao M.R., Cooke M.S., Kuo C.Y. Children are particularly vulnerable to environmental tobacco smoke exposure: Evidence from biomarkers of tobacco-specific nitrosamines, and oxidative stress. Environ Int. 2018;120:238–245. doi: 10.1016/j.envint.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Northrup T.F., Khan A.M., Jacob P., 3rd Thirdhand smoke contamination in hospital settings: Assessing exposure risk for vulnerable paediatric patients. Tob Control. 2016;25:619–623. doi: 10.1136/tobaccocontrol-2015-052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas J.L., Guo H., Carmella S.G. Metabolites of a tobacco-specific lung carcinogen in children exposed to secondhand or thirdhand tobacco smoke in their homes. Cancer Epidemiol Biomarkers Prev. 2011;20:1213–1221. doi: 10.1158/1055-9965.EPI-10-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benowitz N.L., Nardone N., Jain S. Comparison of urine 4-(Methylnitrosamino)-1-(3)Pyridyl-1-Butanol and Cotinine for assessment of active and passive smoke exposure in urban adolescents. Cancer Epidemiol Biomarkers Prev. 2018;27:254–261. doi: 10.1158/1055-9965.EPI-17-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassem N.O., Daffa R.M., Liles S. Children's exposure to secondhand and thirdhand smoke carcinogens and toxicants in homes of hookah smokers. Nicotine Tob Res. 2014;16:961–975. doi: 10.1093/ntr/ntu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramírez N., Özel M.Z., Lewis A.C., Marcé R.M., Borrull F., Hamilton J.F. Exposure to nitrosamines in thirdhand tobacco smoke increases cancer risk in non-smokers. Environ Int. 2014;71:139–147. doi: 10.1016/j.envint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Hang B., Sarker A.H., Havel C. Thirdhand smoke causes DNA damage in human cells. Mutagenesis. 2013;28:381–391. doi: 10.1093/mutage/get013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson S.P. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai R., Shen H., Hang B. Exposure to thirdhand smoke results in oxidative damage, increased apoptosis, and altered differentiation in fetal lung fibroblasts. J Investig Mede. 2017;65:119. [Google Scholar]
- 48.Dhall S., Alamat R., Castro A. Tobacco toxins deposited on surfaces (third hand smoke) impair wound healing. Clin Sci (Lond). 2016;130:1269–1284. doi: 10.1042/CS20160236. [DOI] [PubMed] [Google Scholar]
- 49.Sarker A.H., Chatterjee A., Williams M. NEIL2 protects against oxidative DNA damage induced by sidestream smoke in human cells. PLoS One. 2014;9:e90261. doi: 10.1371/journal.pone.0090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedberg E.C., Walker G.C., Siede W. 2nd ed. ASM Press; Washington DC: 2006. DNA Repair and Mutagenesis. [Google Scholar]
- 51.Xu B., Chen M., Yao M. Metabolomics reveals metabolic changes in male reproductive cells exposed to thirdhand smoke. Sci Rep. 2015;5:15512. doi: 10.1038/srep15512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bahl V., Johnson K., Phandthong R., Zahedi A., Schick S.F., Talbot P. From the cover: Thirdhand cigarette smoke causes stress-induced mitochondrial hyperfusion and alters the transcriptional profile of stem cells. Toxicol Sci. 2016;153:55–69. doi: 10.1093/toxsci/kfw102. [DOI] [PubMed] [Google Scholar]
- 53.Hecht S.S. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lao Y., Yu N., Kassie F., Villalta P.W., Hecht S.S. Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2007;20:235–245. doi: 10.1021/tx060207r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson L.A. Formation, repair, and genotoxic properties of bulky DNA adducts formed from tobacco-specific nitrosamines. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/284935. pii: 284935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castonguay A., Lin D., Stoner G.D. Comparative carcinogenicity in A/J mice and metabolism by cultured mouse peripheral lung of N'-nitrosonornicotine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, and their analogues. Cancer Res. 1983;43:1223–1229. [PubMed] [Google Scholar]
- 57.Hang B., Iavarone A., Havel C. 247th National Meeting of the American Chemical Society (ACS) with press release; March 16-20. 2014. NNA, a thirdhand smoke constituent, induces DNA damage in vitro and in human cells. Dallas, TX. [Google Scholar]
- 58.Zhang S., Qiao S., Chen M., Xia Y., Hang B., Cheng S. A investigation of thirdhand smoke pollution in 3 types of places of Nanjing, 2014. Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49:31–35. [in Chinese] [PubMed] [Google Scholar]
- 59.Hang B., Snijders A.M., Huang Y. Early exposure to thirdhand cigarette smoke affects body mass and the development of immunity in mice. Sci Rep. 2017;7:41915. doi: 10.1038/srep41915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hang B., Mao J.H., Snijders A.M. Genetic susceptibility to thirdhand smoke induced lung cancer development. Nicotine Tob Res. 2019;21:1294–1296. doi: 10.1093/ntr/nty127. [DOI] [PubMed] [Google Scholar]
- 61.Mao J.H., Langley S.A., Huang Y. Identification of genetic factors that modify motor performance and body weight using collaborative cross mice. Sci Rep. 2015;5:16247. doi: 10.1038/srep16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang H., Han J., Zhong C., Snijders A.M., Mao J.H. Unsupervised transfer learning via multi-scale convolutional sparse coding for biomedical applications. IEEE Trans Pattern Anal Mach Intell. 2018;40:1182–1194. doi: 10.1109/TPAMI.2017.2656884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drehmer J.E., Walters B.H., Nabi-Burza E., Winickoff J.P. Guidance for the clinical management of thirdhand smoke exposure in the child health care setting. J Clin Outcomes Manag. 2017;24:551–559. [PMC free article] [PubMed] [Google Scholar]
- 64.Northrup T.F., Jacob P., 3rd, Benowitz N.L. Thirdhand smoke: State of the science and a call for policy expansion. Public Health Rep. 2016;131:233–238. doi: 10.1177/003335491613100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samet J.M., Chanson D., Wipfli H. The challenges of limiting exposure to THS in vulnerable populations. Curr Environ Health Rep. 2015;2:215–225. doi: 10.1007/s40572-015-0060-1. [DOI] [PubMed] [Google Scholar]