Figure 3.
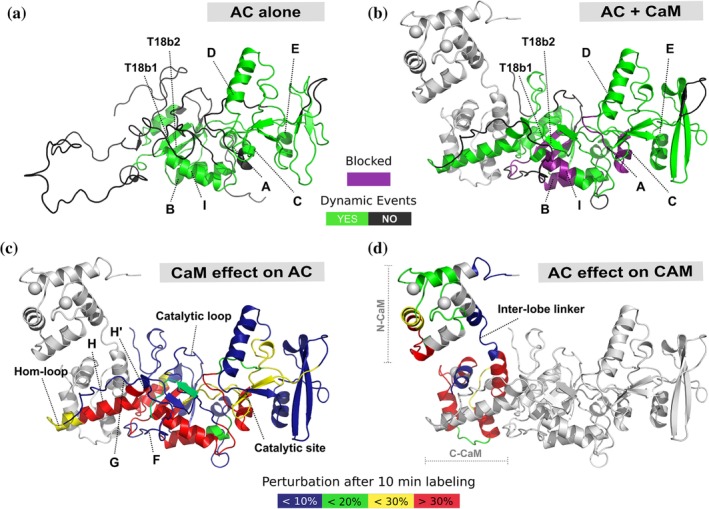
Ligand induced disorder to order transition is critical for catalytic function in CyaA. The structure of free CyaA (AC) eluded previous structural characterization as large sections of the protein are intrinsically disordered. By HDX‐MS it was observed that a 75 amino acid long region, previously seen as helical in the ligand bound structure, was intrinsically disordered in the apo state and becomes ordered upon binding with CaM (a and b). Purple indicates helices with dramatic increases in protection from deuteration, and green indicates regions where a dynamic event occurred upon ligand binding. Differences in percent deuteration on AC and CaM upon binding CaM and AC, respectively, mapped onto the crystal structure (c and d). These data highlight how CaM induces formation of structure in AC upon binding, however that structure is highly dynamic across the H helicies. In contrast the catalytic site remains largely unperturbed as it is primed for high catalytic turnover. Source: Adapted from Reference 52 with permission
