Figure 4. Ubiquitin conjugation regulates NF-κB activation.
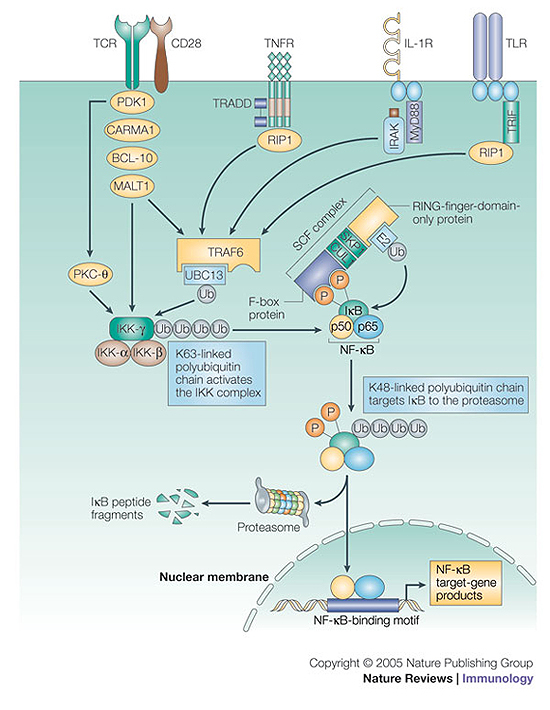
Triggering of various cell-surface receptors leads to assembly of a multimolecular complex that includes the RING (really interesting new gene)-type E3 ligase TRAF6 (tumour-necrosis-factor receptor (TNFR)-associated factor 6). TRAF6 recruits the ubiquitin-loaded E2 ubiquitin-conjugating enzyme 13 (UBC13) and promotes K63 (Lys63)-linked polyubiquitylation of IKK-γ (inhibitor of NF-κB (IκB) kinase-γ) and activation of the IKK complex. Phosphorylation of IκB by the IKK complex recruits an SCF complex (S-phase kinase-associated protein 1 (SKP1)–cullin-1 (CUL1)–F-box-protein complex), which is an E3 ligase that induces K48-linked polyubiquitylation of IκB. This results in the proteasome-dependent degradation of IκB and the release of NF-κB (nuclear factor-κB), which is required for the transactivation of genes by NF-κB. BCL-10, B-cell lymphoma 10; CARMA1, caspase-recruitment domain (CARD)–membrane-associated guanylate kinase (MAGUK) protein 1; IL-1R, interleukin-1 receptor; IRAK, IL-1R-associated kinase; MALT1, mucosa-associated-lymphoid-tissue lymphoma-translocation gene 1; MyD88, myeloid differentiation primary-response protein 88; PDK1, 3-phosphoinositide-dependent protein kinase 1; PKC-θ, protein kinase C-θ; RIP1, receptor-interacting protein 1; TCR, T-cell receptor; TLR, Toll-like receptor; TRADD, TNFR-associated via death domain; TRIF, Toll/IL-1R (TIR)-domain-containing adaptor protein inducing interferon-β.
