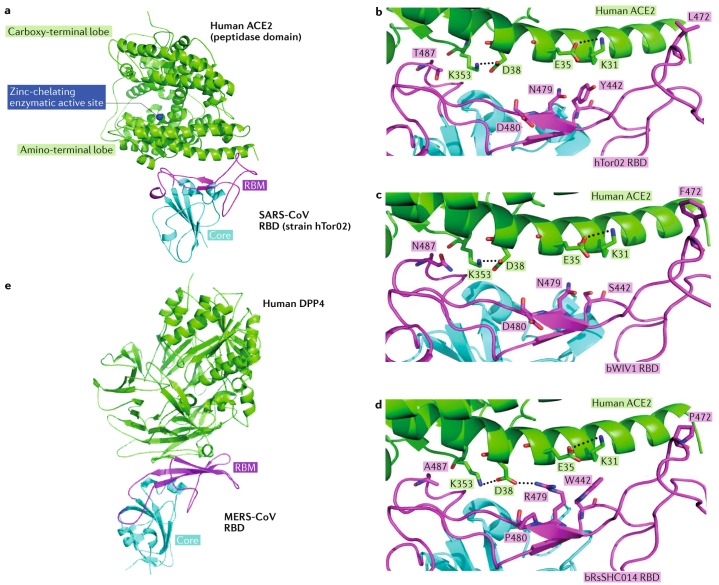
Fig. 6. Receptor recognition by SARS-CoV and MERS-CoV.
a | Severe acute respiratory syndrome coronavirus (SARS-CoV) uses its receptor-binding domain (RBD) (as shown in the structure of strain hTor02, containing core structure (cyan) and receptor-binding motif (RBM; magenta)) to bind human angiotensin-converting enzyme 2 (ACE2; green; Protein Data Bank ID: 2AJF). ACE2 is a peptidase with zinc (blue) in its active centre. b | Several residues in the host and viral receptor, as well as two salt bridges that stabilize the structure (dotted lines) and form two binding hot spots, are crucial for binding of the severe acute respiratory syndrome (SARS) epidemic strain hTor02. Hydrophobic residues surrounding the two salt bridges are present in the structure but are not shown in the figure. c | By contrast, the SARS-related coronavirus (SARSr-CoV) strain bWIV1, which was isolated from bats and can infect both civet and human cells, differs in residues 442, 472 and 487. The mutation from threonine to asparagine in residue 487 introduces a polar side chain and is predicted to interfere with binding at hot spot 353. The model shown here was built on the basis of the structure of hTor02 RBD complexed with human ACE2 (Protein Data Bank ID: 2AJF), in which residues 442, 472 and 487 were mutated from those in strain hTor02 to those in strain bWIV1. d | The bat SARSr-CoV strain bRsSHC014 can also infect human and civet cells; it carries an alanine in position 487, and the short side chain of this residue does not support the structure of hot spot 353. The model was built on the basis of the structure of cOptimize RBD complexed with human ACE2 (Protein Data Bank ID: 3SCJ), in which residues 442, 480 and 487 were mutated from those in strain cOptimize to those in strain bWIV1. e | The Middle East respiratory syndrome coronavirus (MERS-CoV) RBD (core structure in cyan and RBM in magenta) binds human dipeptidyl peptidase 4 (DPP4; green; Protein Data Bank ID: 4KR0). Structure figures were made using PyMOL115. Modelled mutations in panels c and d were performed using Coot140. Panels a–d are adapted from ref.83: this research was originally published in The Journal of Biological Chemistry. Wu, K. L., Peng, G. Q., Wilken, M., Geraghty, R. J. & Li, F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2012; 287:8904–8911. © American Society for Biochemistry and Molecular Biology.