Figure 1. DC-SIGN signalling modulates TLR signalling through RAF1-dependent acetylation of p65.
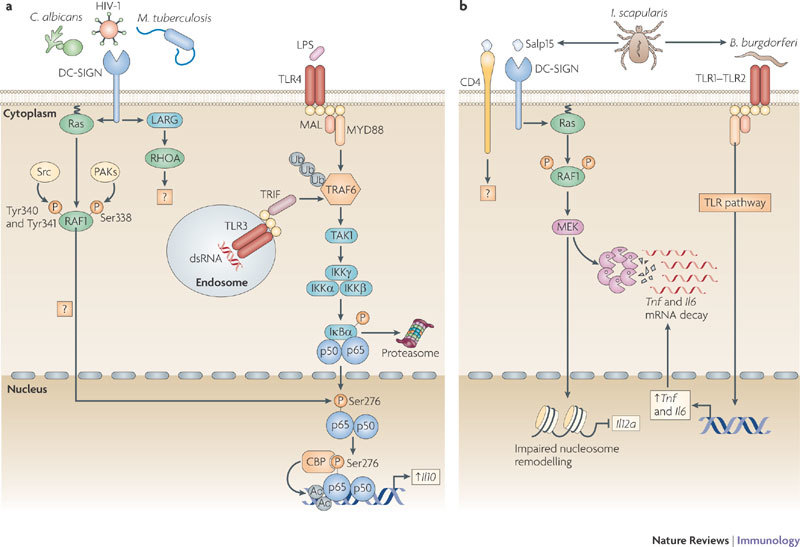
a | DC-specific ICAM3-grabbing non-integrin (DC-SIGN) binding to pathogens such as HIV-1, Mycobacterium tuberculosis and Candida albicans activates the small GTPase Ras proteins, which associate with the serine/threonine protein kinase RAF1 to allow its phosphorylation at residues Ser338, and Tyr340 and Tyr341 by p21-activated kinases (PAKs) and Src kinases, respectively. The upstream effectors that activate PAKs and Src kinases are unknown but might involve leukaemia-associated Rho guanine nucleotide exchange factor (LARG) and Ras homologue A (RHOA), as these proteins are activated by HIV-1 binding to DC-SIGN. RAF1 activation leads to modulation of Toll-like receptor (TLR)-induced nuclear factor-κB (NF-κB) activation. TLR3 and TLR4 activation by double-stranded RNA (dsRNA) and lipopolysaccharide (LPS), respectively, leads to the recruitment of the ubiquitin ligase TNF receptor-associated factor 6 (TRAF6) by myeloid differentiation primary response protein 88 (MYD88), TIR-domain-containing adaptor protein inducing IFNβ (TRIF) and other factors (not depicted). Self-induced polyubiquitylation (Ub) of TRAF6 mediates the recruitment of TGFβ-activating kinase 1 (TAK1), which activates the IκB kinase (IKK) complex. IKKβ phosphorylates inhibitor of NF-κBα (IκBα), thereby targeting it for proteasomal degradation and releasing NF-κB from inhibition, which then translocates into the nucleus, where it binds to NF-κB sites in the enhancer and promoter regions of target genes. RAF1 induces the phosphorylation of p65 at Ser276 through an unknown pathway. Phosphorylated Ser276 serves as a binding site for the histone acetyltransferases CREB-binding protein (CBP) and p300 (not depicted) to acetylate (Ac) p65 at different lysines. Acetylated p65 exhibits enhanced transcriptional activity as well as prolonged nuclear presence owing to impaired binding by IκB, which increases the rate of Il10 (interleukin-10) transcription and prolongs the binding of NF-κB to the Il10 promoter, thereby increasing the production of IL-10. b | Binding of the salivary protein Salp15 from the tick Ixodes scapularis to DC-SIGN activates RAF1, but co-ligation with another receptor, presumably CD4, changes downstream effectors of RAF1, leading to MEK (MAPK/ERK kinase) but not ERK (extracellular signal-regulated kinase) activation. The spirochete Borrelia burgdorferi exploits the effects of Salp15 to infect humans. Salp15-induced MEK-dependent signalling decreases B. burgdorferi-induced TLR1–TLR2-dependent pro-inflammatory cytokine production by enhancing the decay of Il6 and Tnf (tumour necrosis factor) mRNA while impairing nucleosome remodelling at the Il12a promoter, which is required for transcriptional initiation. MAL, MYD88-adaptor-like protein.
