Abstract
Antibodies are a crucial part of the body's specific defense against infectious diseases and have considerable potential as therapeutic and prophylactic agents in humans and animals. The development of recombinant single-chain antibodies allows a genetic application strategy for prevention of infectious diseases. To test this in a fish model, a gene construct encoding a neutralizing single-chain antibody to the fish-pathogenic rhabdovirus VHSV (viral hemorrhagic septicemia virus) was administered to rainbow trout by intramuscular injection of plasmid DNA. Circulating recombinant antibodies could later be detected in the fish, and protective immunity to the viral disease was established.
Main
Use of recombinant single-chain antibodies for prevention or treatment of diseases has been addressed in various studies including neutralization of viruses1,2 and toxins3, cancer immunotherapy4,5, and recently also in the form of DNA vaccines encoding anti-idiotype single-chain antibodies6. At cell culture level, expression of virus-specific single-chain antibodies inside host cells can interfere with virus multiplication within the cell7,8. The present study aimed to establish protection against an infectious pathogen in vivo by administration of antibody genes. The experimental model involved the fish rhabdovirus viral hemorrhagic septicemia virus (VHSV), with rainbow trout (Oncorhynchus mykiss ) as the host species9,10. As with the morphologically related mammalian rabies virus, studies have identified the viral glycoprotein G as the target of neutralizing antibodies11, and adoptive transfer of immunity can be obtained by intraperitoneal injection of neutralizing monoclonal antibodies (MAbs) before exposure to VHSV12.
Gene constructs encoding single chain antibodies, with the human Ig kappa constant domain fused to the 3′ end (ScAbs) were prepared using cDNA libraries from hybridoma cell lines producing neutralizing antibodies to the VHSV G protein. The variable parts of the immunoglobulin (Ig) heavy and light chain genes were cloned13 and connected by a sequence encoding a 14–amino acid flexible spacer. A constant-domain gene of the human Ig κ-chain was added as a tag at the 3′ end14. Upon expression in bacteria, the resulting ScAbs were able to neutralize VHSV (P. M. C., N. L. et al. unpublished data). Recombinant single-chain neutralizing antibodies have also been reported for several important mammalian viruses, including HIV-1 (ref. 15), rabies virus1, and vesicular stomatitis virus (VSV)16. In the case of VSV, protective activity could furthermore be demonstrated in vivo, but because of the short half-life of the single-chain antibody molecules in circulation, protection was obtained only when the antibody was allowed to interact with the virus in vitro before inoculation of the mice16. Short half-life of neutralizing single-chain antibodies administered to mice before infection was correspondingly used to explain the lack of protection against exposure to a lethal coronavirus17. Here, we aimed at maintaining a stable level of circulating ScAb within the host over a longer period of time by inducing expression in vivo through administration of a plasmid-borne recombinant antibody gene, rather than delivery of the antibody itself. Such a strategy has earlier been suggested for cancer immunotherapy4 but has to our knowledge not been reported as a prophylactic measure.
Results and discussion
Expression of antibody in vitro.
A ScAb variant based on the variable-domain genes from the hybridoma cell line producing neutralizing monoclonal antibody 3F1H10 (refs 12,13) was selected for the experiments. To mediate secretion in fish cells, the gene sequence encoding the signal peptide of rainbow trout transforming growth factor β (TGF-β; ref. 18 ) was included at the 5′ end of the ScAb gene. The resulting construct (BU1) was inserted into a eukaryotic expression plasmid (pcDNA3) to give pcDNA3-BU1 (Fig. 1).
Figure 1. Schematic drawing of the pcDNA3-BU1 plasmid used for expression studies in vitro and in vivo.
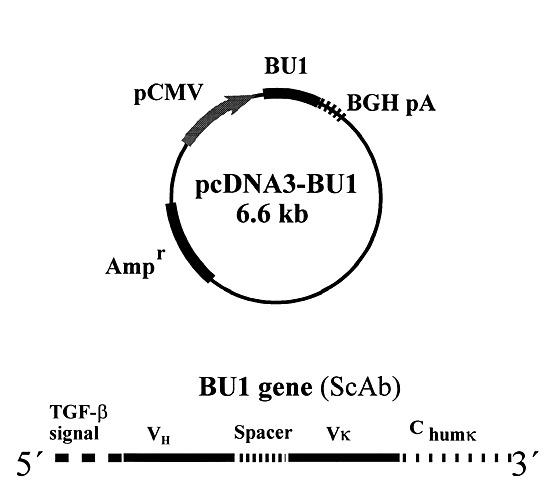
pCMV, cytomegalovirus promoter; BGH pA, bovine growth hormone polyadenylation signal; Ampr, gene encoding ampicillin resistance; TGF-β, trout transforming growth factor β; VH, Vκ, variable-domain regions from hybridoma Ig heavy- and κ-light chain genes, respectively; Chum κ, constant part of human Ig κ-light chain gene.
In order to examine the protective effect of this construct in vitro, monolayers of fish cell cultures were transfected with purified plasmid DNA and five days later exposed to live VHSV. When examined by immunofluorescence after three to five days, cultures transfected with pcDNA3-BU1 were either uninfected or the infection was at an early stage with plaques of infected cells, compared to the generalized infection and destruction of the cell layer found in cultures transfected with the vector without insert (Fig. 2). Single cells expressing ScAb could be detected in cultures transfected with pcDNA3-BU1 (Fig. 2A), but no such cells were present in cultures transfected with pcDNA3 (Fig. 2B). Virus-neutralizing activity with titers up to 32 in a plaque neutralization test12 could correspondingly be detected in supernatants from cell cultures transfected with pcDNA3-BU1, whereas no neutralizing activity (titers below 2) was found in supernatants from cultures transfected with pcDNA3. Exchange of the cell culture medium shortly before inoculation with VHSV eliminated the protective effect of transfection with pcDNA3-BU1 (data not shown). The secreted ScAbs were thus likely to be responsible for the observed interference with virus propagation in the cell culture.
Figure 2. Inhibition of VHSV in cell cultures secreting ScAb.
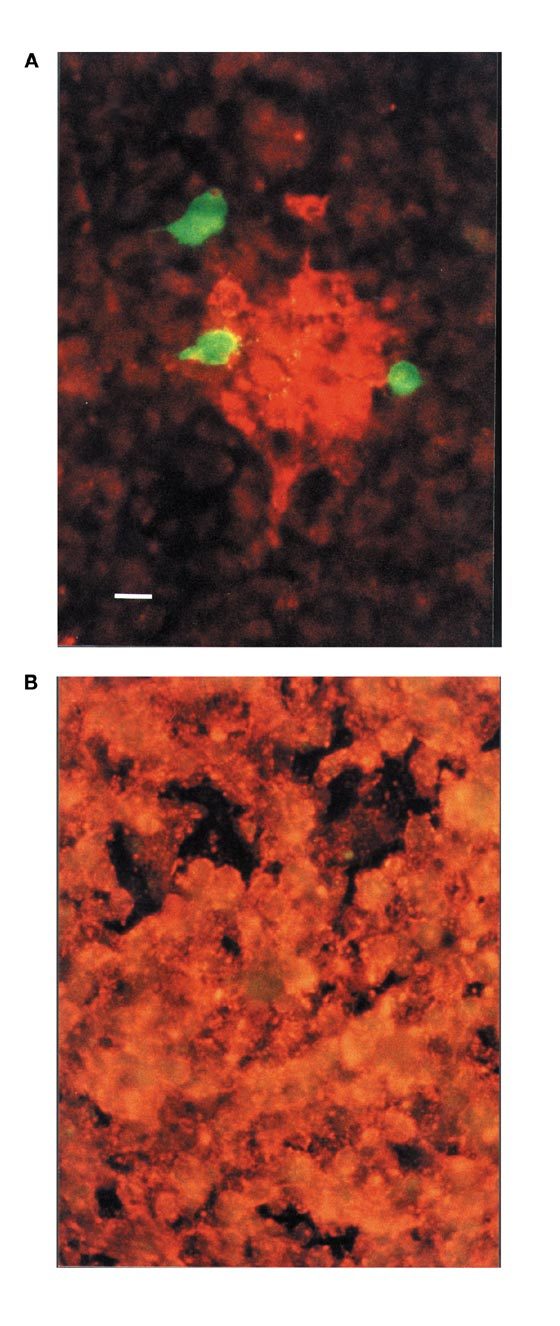
Cultures of fish cells were transfected with pcDNA3-BU1 (A) or pcDNA3 plasmid DNA (B) before inoculation with VHSV. Three days after inoculation the cultures were fixed and stained by dual-color immunofluorescence. Cells expressing ScAb were detected by using FITC-conjugated antibody (green fluorescence). Virus-infected cells were detected by TRITC-conjugated antibody (orange-red fluorescence). Bar corresponds to 10 μm.
Expression of antibody in vivo.
To test the ScAb gene construct in vivo, we injected rainbow trout fingerlings intramuscularly with 40 μg purified plasmid. The presence of ScAbs could later be demonstrated in muscle cells in tissue from fish injected with pcDNA3-BU1 ( Fig. 3), whereas no ScAbs were detected in the muscles of fish injected with pcDNA3. When plasma samples taken 12 days post injection were analyzed in an enzyme-linked immunosorbent assay (ELISA) for ScAbs recognizing VHSV, a positive signal was obtained only with samples from fish injected with pcDNA3-BU1. With the corresponding ScAb expressed in Escherichia coli as reference, the concentration of ScAb in the trout plasma was estimated to be 0.1–0.3 μg/ml. The same plasma samples were tested in a plaque neutralization assay12. Samples from 10 individuals injected with pcDNA3-BU1 all neutralized VHSV with titers ranging between 160 and 640, whereas no neutralizing activity was detected in plasma from 10 fish injected with the control plasmid. Unlike trout serum or plasma with neutralizing trout immunoglobulin10, the neutralization was independent of complement. To further verify that the observed activity was due to the presence of ScAbs in the plasma, a pool of plasma samples from fish injected with pcDNA3-BU1 was preincubated with rabbit Ig to human Ig κ-chain (Ra-humκ) before testing in the virus neutralization assay. The ScAbs carried the constant part of the human Ig κ-chain as a tag, and preincubation with Ra-humκ accordingly eliminated the neutralizing activity, whereas no effect was observed upon preincubation with normal rabbit serum or with rabbit serum to trout Ig. For comparison, the rabbit serum to trout Ig reduced the neutralizing activity of the serum from a fish immunized with inactivated VHSV more than 30-fold (Table 1). Furthermore, like the ScAb expressed in bacteria, plasma samples from fish injected with pcDNA3-BU1 could neutralize the virulent VHSV DK-3592B isolate, but not a neutralization escape-mutant of that isolate (DK-3592/p2) (data not shown).
Figure 3. Expression of ScAb in fish muscle.
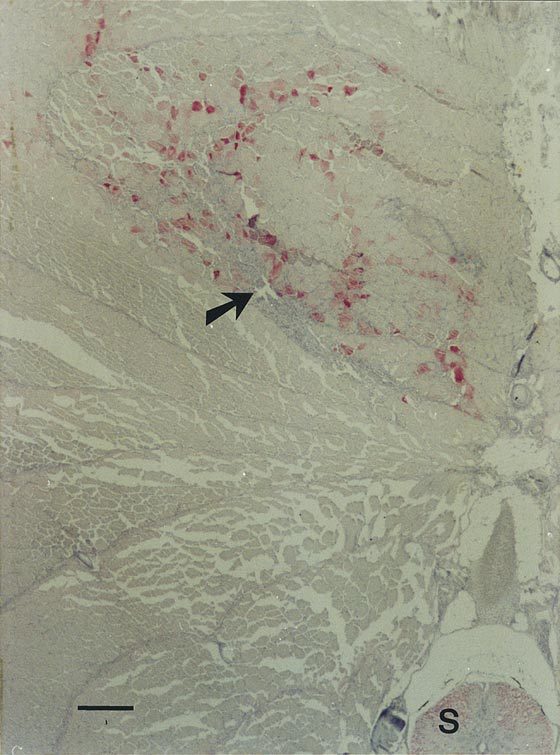
Immunochemical staining of section of epaxial muscle tissue sampled from a fish injected with pcDNA3-BU1 12 days earlier. Presence of ScAb (red staining) was evident in muscle cells along the regenerating needle track (arrow). S, position of the spinal cord. Bar corresponds to 200 μm.
Table 1.
Specific inhibition of neutralizing ScAb in trout plasma
Protection against disease.
When challenged with the VHSV DK-3592B isolate 11 days after injection of plasmid DNA, most of the fish injected with pcDNA3-BU1 survived, whereas high mortalities were observed among fish injected with pcDNA3 (Fig. 4). The specificity of the protection was verified in another experiment including challenge of parallel groups of fish with VHSV DK-3592B, the neutralization escape-mutant DK-3592B/p2 mutant, and a neutralization-resistant wild-type isolate of VHSV. The fish were challenged 18 days post injection with DNA. The pcDNA3-BU1 only provided protection against the 3592B isolate (data not shown).
Figure 4. Development of mortality following exposure to VHSV in groups of fish injected with pcDNA3-BU1 (●) or with pcDNA3 (○) 11 days earlier.
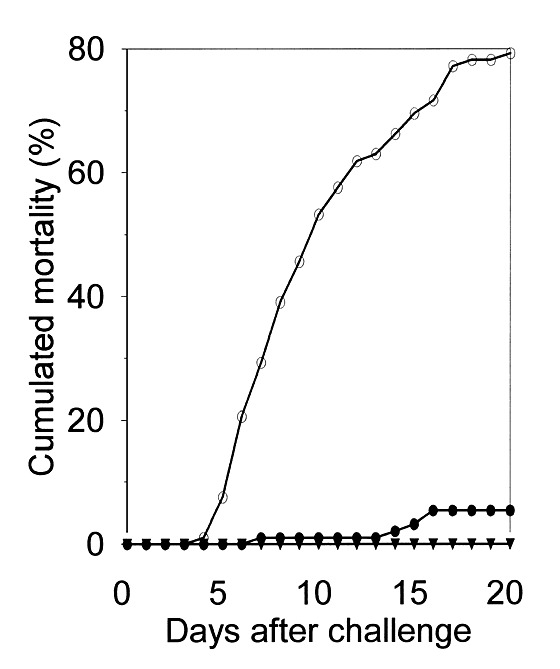
A mock-challenged mixed group of fish was included for determination of background mortality (▴). The graphs represent the mean of three replicate aquariums for each group.
Administration of an antibody gene in the form of naked DNA does not allow intracellular interference with virus multiplication throughout the body as reported for transformed cell cultures7, in that only a few of the animal's cells will take up the plasmids and express the antibody. However, by designing the gene for secretion of the antibody upon expression, we obtained a protective systemic level of recombinant antibody. The use of plasmid DNA circumvents the potentially hazardous use of live recombinant viral vectors often employed for transfer of antibody genes8,19,20. The duration of protection was not analyzed, but between day 18 and day 39 after injection of DNA, the plasma ScAb concentration remained stable in unchallenged fish kept at 16°C. In tissue samples taken at day 39, however, immunohistochemical analyses revealed the beginning of an inflammatory response and degradation of expressing cells at the site of injection, indicating that the ScAb-producing muscle cells were about to be eliminated. In fish transferred to 11°C at day 18, the plasma ScAb level at day 39 was approximately half that in the fish kept at 16°C. Reduced temperature thus appeared to negatively affect the balance between synthesis and clearance rate of the ScAbs. Although single-chain antibodies have low immunogenicity, persistence can probably be improved by use of species-homologous antibody genes. The minimal dose of the pcDNA3-BU1 construct needed to induce protection against VHSV in the fish remains to be determined, but will probably be above the nanogram level of plasmid DNA recently shown to be required for establishment of long-lasting immunity by a traditional DNA vaccine encoding the VHSV G protein21. In aquaculture, future prophylactic use of antibody genes may thus mainly be of practical interest for protection of fish against diseases for which no suitable vaccines are available. The technology may be used not only against viral pathogens as exemplified here by VHSV, but against any infection where immunity can be established by administration of pathogen-specific monoclonal antibodies. One such example is the important “white spot” disease that afflicts a wide range of cultured and ornamental freshwater fish species and is caused by the parasitic ciliate Ichthyopthirius multifiliis22.
From a general veterinary viewpoint, development of transgenics harboring protective antibody genes in their germline may be considered. Such a strategy has already been successfully used in plants23. If delivery of naked DNA carrying antibody genes for immunoprophylaxis can be extended to mammals and to humans in particular, it may provide a valuable tool for transient protection of exposed individuals against pathogens or toxins when vaccination is not possible or not efficient (e.g., malaria, snake venoms). Similarly, it may be possible to induce antibodies of a desired specificity in immunodeficient individuals or in the case of risk of certain infections such as bacterial sepsis or infections with HIV where individuals themselves may tend to produce nonprotective antibodies24,25. Compared to the current practice of clinical administration of antiserum or Ig from human donors or immunized animals, use of plasmid DNA would eliminate the risk of concomitant transfer of infections. A possible drawback is that pathogen variability could overcome the immunity established by a single recombinant mAb, a problem that became evident in the virological examintions of survivors from the experiment illustrated in Figure 4. VHSV was isolated from some of the healthy survivors injected with pcDNA3-BU1, and one of these re-isolations was resistant to neutralization because of an amino acid substitution in the central variable region of the G protein (data not shown). To reduce the risk of insufficient protection, then, it may be preferable to administer either genes encoding a number of single-chain antibodies to different epitopes of the pathogen, or possibly even an entire single-chain antibody gene expression library toward a given pathogen. Other improvements at the molecular level may include incorporation of biological effector functions such as complement activation into the ScAbs. This would be particularly important for protection against pathogens such as Ebola virus where the subclass of the antibodies has appeared to be critical to the efficacy of the protection26.
Experimental protocol
Plasmid constructs.
The ScAb gene BU1 was inserted by blunt-end ligation between the cytomegalovirus (CMV) promoter and the bovine growth hormone polyadenylation signal (BGHpA) at the EcoRI site of the pcDNA3 vector (Invitrogen, Groningen, the Netherlands). In the ScAb gene construct, the variable-domain gene sequences of the Ig heavy chain (VH) and the Ig kappa light chain (Vκ) from the 3F1H10 hybridoma13 were joined by a 42-nucleotide spacer fragment14. The VH domain segment was mutated in two positions giving amino acid substitutions N35T and K64T. These substitutions were based on the VH domain sequence of hybridoma 3F1A2 (ref. 13). Combination of the variable domains of hybridomas 3F1H10 and 3F1A2 was found to increase the neutralizing activity of the ScAb (P.M.C., N.L. et al., unpublished data). The sequence encoding the trout TGF-β secretion signal peptide18 was included at the 5′ end of the gene, and the constant part of the human Ig κ-chain gene (Chum-κ) was included as a tag at the 3′ end14. The insert sequence was verified on an automatic sequencing analyzer (GenBank AF 302092). The pcDNA3 plasmid without insert was used as negative control. Plasmid DNA for transfection and injection was purified from overnight cultures of E. coli DH5-α by the use of commercial kits for anion-exchange chromatography (Qiagen, Hilden, Germany). Other molecular biology procedures followed standard protocols.
Expression of ScAb in transfected cell cultures.
Fish cell cultures (epithelioma papulosum cyprini (EPC)) were transfected with purified plasmids in 24-well cell culture trays as described27. Five days after transfection, 100–1,000 50% tissue culture infective doses of VHSV reference strain F1 (ref. 28) was added to the wells. After incubation at 15°C for three to five days, the culture wells were fixed in 80% cold acetone and stained by dual-color immunofluorescence following previous procedures29. Detection of ScAb-expressing cells was performed with rabbit Ig to human Ig κ-chain (Ra-humκ; DAKO, Glostrup, Denmark) followed by fluorescein isothiocyanate (FITC)–conjugated swine Ig to rabbit Ig (DAKO). Detection of VHSV-infected cells was performed with tetramethylrhodamine isothiocyanate (TRITC)–conjugated mAb IP5B11 recognizing the VHSV nucleocapsid protein29. Micrographs were made by double exposure of an ISO 1600 film using a Leitz DM epifluorescence microscope with filters for excitation of FITC (green emission) and TRITC (red emission), respectively.
Immunohistochemical analysis for expression of ScAb in injected fish.
Twelve days after injection of plasmid DNA, 10 fish were sampled for each plasmid construct. After killing the fish, a transverse section of muscle tissue was excised from the site of injection. The tissue was fixed in 10% phosphate-buffered formalin and analyzed by immunohistochemistry following earlier outlined procedures30. The ScAbs were detected with Ra-humκ as primary antibody and biotinylated swine Ig to rabbit Ig (DAKO) as the secondary antibody.
Serological examination for VHSV-reactive ScAbs.
Blood samples were collected at different time points after injection of plasmid DNA from fish not exposed to VHSV. Sampling was performed with heparin-treated capillary tubes after cutting off the posterior fin of fully anesthetized fish. The fish were killed immediately afterward. After centrifugation at 7,000 g, plasma samples were collected and stored at −80°C until analyzed.
Supernatants from transfected cell cultures as well as plasma samples from DNA-injected fish were examined for anti-VHSV reactive ScAbs by a plaque neutralization assay (50% PNT) with or without complement in the form of normal trout serum12,31 and by ELISA. To analyze whether the virus-neutralizing activity detected in the trout plasma by the 50% PNT assay was due to the ScAbs produced by the fish and not by trout antibodies, a plasma pool from 10 fish injected with pcDNA3-BU1 was preincubated with normal rabbit serum, Ra-humκ, or rabbit serum to trout Ig (final dilution 1/100). Preincubation time was 1 h at room temperature followed by 1 h at 4°C. Following preincubation, VHSV (F25 variant) and complement serum were added, followed by incubation at 4°C overnight before application on fish cell cultures as described earlier31. Reactivity with VHSV in ELISA was examined in 96-well plates coated with purified virus following the procedure earlier outlined for mAbs13, with the modification that horseradish peroxidase (HRP)-conjugated Ra-humκ was used for detection of bound antibody. Quantitative comparisons of plasma ScAb levels were performed using a sandwich ELISA with Ra-humκ as the first layer, followed by dilutions of the plasma samples, and finally HRP-conjugated Ra-humκ for detection of bound ScAb. Washing and other parameters were as described earlier for quantification of mAbs32. As a positive reference, ScAb expressed in E. coli and purified as described by Molloy et al.14 was used.
DNA immunoprotection trials with fish.
Disease-free rainbow trout fingerlings, average weight 4.5 g, were anesthetized with 0.01% benzocaine and each given two simultaneous injections of 20 μg plasmid DNA in 25 μl saline buffer. The injections were performed with a 0.4 × 19 mm needle in the epaxial muscle on each side of the fish. The fish were subsequently kept in groups of 150 individuals in 120 L tanks supplied with running tap water.
Eleven or eighteen days after injection of plasmid DNA, groups of fish were exposed to (challenged with) VHSV by immersion in water containing 2 × 105 50% tissue culture infective doses of virus per milliliter as outlined elsewhere30. Challenge was performed in replicate 8 L aquariums with 25–34 fish in each. Dead fish were recorded and collected daily. Representative samples of dead fish from all tanks were analyzed virologically for the presence of VHSV. Mean water temperature was 16°C from the time of injection to challenge. Before challenge the fish were adapted to 11°C, and this temperature was kept throughout the 20- to 22-day challenge period.
Acknowledgements
We thank L. Troels, H. Hermansen, and T.E. Kjaer for technical assistance. S.E. LaPatra, K.B. Pedersen, and M. Madsen are thanked for review of the manuscript. L. Knudsen is thanked for assistance with typing. This work was supported by the European Commission (Contract FAIR CT 95-0666) and by the Danish Ministry of Food, Agriculture and Fisheries (Contract Fisk 97-2).
References
- 1.Muller BH. Phage-displayed and soluble mouse scFv fragments neutralize rabies virus. J. Virol. Methods. 1997;67:221–233. doi: 10.1016/S0166-0934(97)00099-2. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto M. Intracellular single-chain antibody against Hepatitis B virus core protein inhibits the replication of Hepatitis B virus in cultured cells . Hepatology. 1999;30:300–307 . doi: 10.1002/hep.510300105. [DOI] [PubMed] [Google Scholar]
- 3.Mousli M, Devaux C, Rochat H, Goyffon M, Billiald P. A recombinant single-chain antibody fragment that neutralizes toxin II from the venom of the scorpion Androctonus australis hector. FEBS Lett. 1999;442:183– 188. doi: 10.1016/S0014-5793(98)01647-0. [DOI] [PubMed] [Google Scholar]
- 4.Nicolet CM. Expression of a tumor-reactive antibody-interleukin 2 fusion protein after in vivo particle-mediated gene delivery. Cancer Gene Ther. 1995;2:171–170. [PubMed] [Google Scholar]
- 5.Cochet O. Intracellular expression of an antibody fragment-neutralizing p21 ras promotes tumor regression. Cancer Res. 1998;58: 1170–1176. [PubMed] [Google Scholar]
- 6.King CA. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat. Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- 7.Marasco WA, Haseltine WA, Chen S. Design, intracellular expression, and activity of a human anti-human immunodeficiency virus type 1 gp120 single-chain antibody . Proc. Natl. Acad. Sci. USA. 1993;90:7889– 7893. doi: 10.1073/pnas.90.16.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BouHamdan M, Duan L-X, Pomerantz RJ , Strayer DS. Inhibition of HIV-1 by an anti-integrase single-chain variable fragment (SFv): delivery by SV40 provides durable protection against HIV-1 and does not require selection. Gene Ther. 1999;6:660– 666. doi: 10.1038/sj.gt.3300864. [DOI] [PubMed] [Google Scholar]
- 9.Lenoir G, de Kinkelin P. Fish rhabdoviruses: comparative study of protein structure. J. Virol. 1975;16 :259–262. doi: 10.1128/jvi.16.2.259-262.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzen N, Olesen NJ, Koch C. Immunity to VHS virus in rainbow trout. Aquaculture. 1999;172:41–61. doi: 10.1016/S0044-8486(98)00443-8. [DOI] [Google Scholar]
- 11.Lafon M. Lyssaviruses. 1994. Immunobiology of Iyssaviruses: the basis for immunoprotection; pp. 145–160. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzen N, Olesen NJ, Jørgensen PEV. Neutralisation of Egtved virus pathogenicity to cell cultures and fish by monoclonal antibodies to the viral G protein . J. Gen. Virol. 1990;71:561– 567. doi: 10.1099/0022-1317-71-3-561. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzen N, Cupit PM, Secombes CJ , Cunningham C. Three monoclonal antibodies to the VHS virus glycoprotein: comparison of reactivity in relation to differences in immunoglobulin variable domain gene sequences . Fish Shellfish Immunol. 2000;10:129– 142. doi: 10.1006/fsim.1999.0232. [DOI] [PubMed] [Google Scholar]
- 14.Molloy P, Brydon L, Porter AJ , Harris WJ. Separation and concentration of bacteria with immobilized antibody fragments . J. Appl. Bacteriol. 1995;78:359– 365. doi: 10.1111/j.1365-2672.1995.tb03418.x. [DOI] [PubMed] [Google Scholar]
- 15.Srikantan V. Cloning and biological characterization of human single-chain Fv fragments that mediate neutralization of HIV-1. AIDS. 1994; 8:1525–1532. doi: 10.1097/00002030-199411000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kalinke U. Monovalent single-chain Fv fragments and bivalent miniantibodies bound to vesicular stomatitis virus protect against lethal infection. Eur. J. Immunol. 1996;26:2801–2806. doi: 10.1002/eji.1830261202. [DOI] [PubMed] [Google Scholar]
- 17.Lamarre A, Yu MWN, Chagnon F, Talbot PJ. A recombinant single chain antibody neutralizes coronavirus infectivity but only slightly delays lethal infection of mice. Eur. J. Immunol. 1997;27 :3447–3455. doi: 10.1002/eji.1830271245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardie LJ. Isolation of the first piscine transforming growth factor beta gene: analysis reveals tissue specific expression and a potential regulatory sequence in rainbow trout (Oncorhynchus mykiss) Cytokine . 1998;10:555–563. doi: 10.1006/cyto.1997.0334. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Venugopal K, Gould EA. Intracellular interference of tick-borne Flavivirus infection by using a single-chain antibody fragment delivered by recombinant Sindbis virus. J. Virol. 1995;69:1044– 1049. doi: 10.1128/jvi.69.2.1044-1049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittington HA, Ashworth LJ, Hawkins RE. Recombinant adenoviral delivery for in vivo expression of scFv antibody fusion proteins. Gene Ther. 1998; 5:770–777. doi: 10.1038/sj.gt.3300685. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzen, E., Einer-Jensen, K., Martinussen, T., LaPatra, S.E. & Lorenzen, N. DNA vaccination of rainbow trout against viral hemorrhagic septicemia virus: a dose-response and time-course study. J. Aquat. Anim. Health, in press (2000).
- 22.Lin T, Clark TG, Dickerson HW. Passive immunization of channel catfish (Ictalurus punctatus) against the ciliated protozoan parasite Ichthyophthirius multifiliis by use of murine monoclonal antibodies. Infect. Immun. 1996;64:4085–4090. doi: 10.1128/iai.64.10.4085-4090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavladoraki P. Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature. 1993; 366:469–472. doi: 10.1038/366469a0. [DOI] [PubMed] [Google Scholar]
- 24.Cross AS. Immunotherapy of sepsis: flawed concept or faulty implementation? . Vaccine. 1999;17:13–21. doi: 10.1016/S0264-410X(99)00230-3. [DOI] [PubMed] [Google Scholar]
- 25.Soudeyns H, Pantaleo G. The moving target: mechanisms of HIV persistence during primary infection. Immunol. Today. 1999;20:446–450. doi: 10.1016/S0167-5699(99)01504-2. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JA. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664– 1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 27.Heppell J. Development of DNA vaccines for fish: vector design, intra muscular injection and antigen expression using viral haemorrhagic septicaemia virus genes as model. Fish Shellfish Immunol. 1998;8: 271–286. doi: 10.1006/fsim.1997.0133. [DOI] [Google Scholar]
- 28.Jensen MH. Research on the virus of Egtved disease. Ann. N.Y. Acad. Sci. 1965; 126:442–446. doi: 10.1111/j.1749-6632.1965.tb14292.x. [DOI] [PubMed] [Google Scholar]
- 29.Jørgensen PEV, Olesen NJ, Lorenzen N, Winton JR, Ristow S. Infectious hematopoietic necrosis (IHN) and viral haemorrhagic septicaemia (VHS): detection of trout antibodies to the causative viruses by means of plaque neutralization, immunofluorescence and enzyme-linked immunosorbent assay. J. Aquat. Anim. Health. 1991; 3:100–108. doi: 10.1577/1548-8667(1991)003<0100:IHNIAV>2.3.CO;2. [DOI] [Google Scholar]
- 30.Lorenzen N. Protective immunity to VHS in rainbow trout (Oncorhynchus mykiss, Walbaum) following DNA vaccination. Fish Shellfish Immunol. 1998;8:261–270. doi: 10.1006/fsim.1997.0134. [DOI] [PubMed] [Google Scholar]
- 31.Olesen NJ, Jørgensen PEV. Detection of neutralizing antibody to Egtved virus in rainbow trout (Salmo gairdneri) by plaque neutralization test with complement addition. J. Appl. Ichthyol. 1986;2:33–41. doi: 10.1111/j.1439-0426.1986.tb00427.x. [DOI] [Google Scholar]
- 32.Lorenzen N, Olesen NJ, Jørgensen PEV. Production and characterization of monoclonal antibodies to four Egtved virus structural proteins. Dis. Aquat. Org. 1988;4:35–42. doi: 10.3354/dao004035. [DOI] [Google Scholar]
- 33.Lorenzen N, LaPatra SE. Immunity to rhabdoviruses in rainbow trout: the antibody response. Fish Shellfish Immunol. . 1999;9:345–360. doi: 10.1006/fsim.1999.0194. [DOI] [PubMed] [Google Scholar]


