Figure 1. Complex interactions among the disease, the immune system and immunomodulatory biologics that influence safety and efficacy.
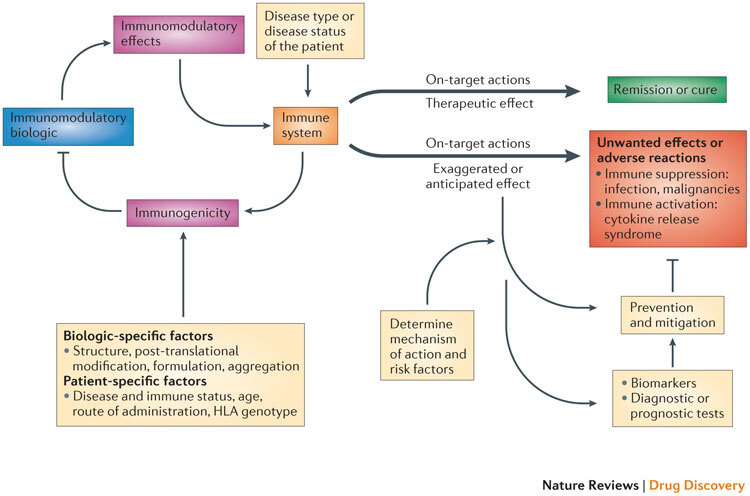
The interaction of the immunomodulatory biologic with the immune system and immune processes results in either the required (or intended) on-target therapeutic effect or unwanted reactions. Adverse reactions such as unwanted immunosuppression or immune activation are usually associated with the on-target exaggerated pharmacology of the biologic (for example, immunosuppression from a tumour necrosis factor (TNF)-specific therapy increases the risk of reactivation of tuberculosis or there is the risk of inducing cytokine release syndrome through the excessive activation of T cells with muromonab-CD3 therapy). The biologic also has the potential to induce a host immune response (termed immunogenicity), which results in the formation of drug-targeting antibodies that in turn can impede the therapeutic efficacy of the immunomodulatory biologic. The disease type and status of the patient can also influence the functional state of the immune system and thereby determine whether the interaction with the biologic leads to a therapeutic effect or unwanted adverse reactions (for example, chronic inflammation associated with diseases such as rheumatoid arthritis exposes the patient to an increased risk of malignancy). Other patient-specific factors such as human leukocyte antigen (HLA) type as well as the route and frequency of administration have a bearing on the propensity to develop immunogenicity to the biologic, as these factors contribute to antigen processing and presentation of immunogenic epitopes. Factors that are intrinsic to the property of the biologic (biologic-specific factors), such as the presence of immunogenic epitopes, glycosylation and aggregation, also affect the generation of an immunogenic response. The prevention and mitigation of these unwanted adverse reactions is predicated on a detailed knowledge and understanding of the mechanisms and risk factors that drive the adverse reactions and the use of effective biomarkers and diagnostic tests. For example, knowledge of the association of the John Cunningham virus (JCV) in the aetiopathology of progressive multifocal leukoencephalopathy (PML) observed in patients receiving natalizumab (Tysabri; Biogen Idec/Elan) led to the recognition of the presence of JCV as a risk factor for PML. Consequently, a diagnostic test for JCV seropositivity is now used to stratify patients before initiating natalizumab therapy.
