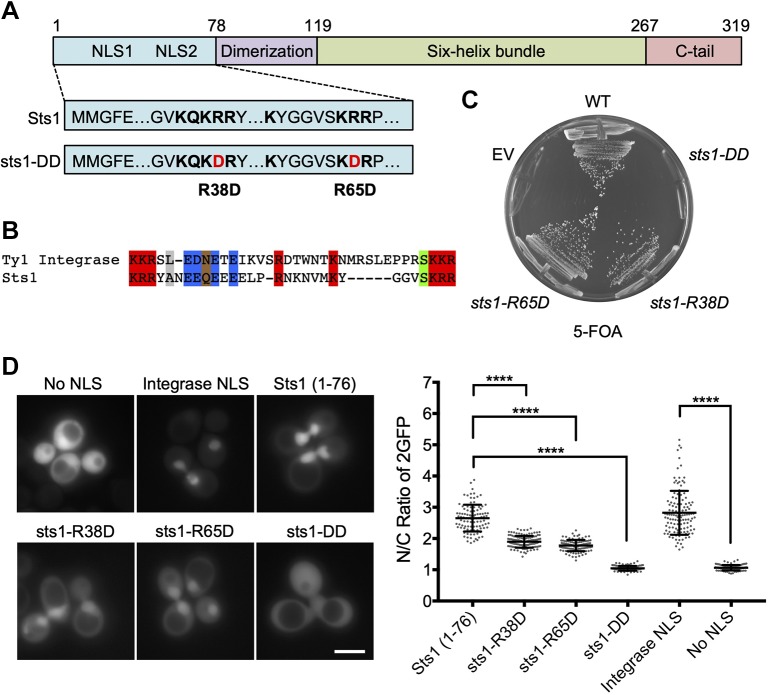
Fig. 1.
Sts1 contains an apparent bipartite NLS essential for cell viability. (A) Predicted domain architecture and selected functional elements of Sts1 based on sequence analysis and comparison with the crystal structure of the S. pombe homolog Cut8. The suggested NLS elements are indicated with the sequences shown below; the two mutations in the sts1-DD mutant are also shown. (B) Sts1 lacks a strong match to canonical NLS, but has substantial similarity to a confirmed bipartite NLS in the Ty1 integrase; in both cases the linker separating the two basic elements is unusually long. (C) Viability assay of Sts1 NLS mutants. The noted sts1 alleles, expressed under the endogenous STS1 promoter and terminator from pRS314-based plasmids, were transformed into MHY9580 yeast, in which the chromosomal sts1Δ allele is covered by pRS316-STS1. Transformed cells were struck on 5-FOA plates to evict the cover plasmid. EV, empty vector; WT, wild-type. (D) WT MHY500 yeast transformed with MET25 promoter-based plasmids expressing the indicated NLS sequences fused to 2GFP. Sts1 constructs expressed Sts1 residues 1–76 appended to the N-terminus of 2GFP. The NLS sequence from Ty1 integrase N-terminally tagged with 2GFP was used as a positive control for a bipartite NLS, and 2GFP without an NLS (‘No NLS’) was used as a negative control (both obtained from Anita Corbett). Transformants were grown to mid-log phase at 30°C prior to fluorescence imaging. Three biological replicates of at least 100 cells each were counted (right panel). A t-test was used to determine statistical significance of localization differences (****P<0.0001). Scale bar: 5 μm.