Figure 3. Receptor activation or ligand–receptor complex assembled by type I, type II or type III interferons.
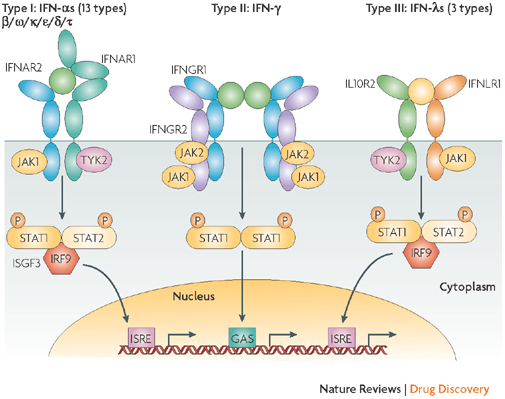
Type I interferons (IFNs) (α, β ω, κ, ɛ, δ (pigs), τ (ruminants)) interact with IFN (α, β and ω) receptor 1 (IFNAR1) and IFNAR2; type II IFN-γ with IFN-γ receptor 1 (IFNGR1) and IFNGR2; and type III IFN-λs with IFN-λ receptor 1 (IFNLR1; also known as IL28RA) and interleukin 10 receptor 2 (IL10R2; also known as IL10RB). Type II IFN-γ is an antiparallel homodimer exhibiting a two-fold axis of symmetry. It binds two IFNGR1 receptor chains, assembling a complex that is stabilized by two IFNGR2 chains. These receptors are associated with two kinases from the JAK family: JAK1 and TYK2 for type I and III IFNs; JAK1 and JAK2 for type II IFN. All IFN receptor chains belong to the class 2 helical cytokine receptor family, which is defined by the structure of the extracellular domains of their members: approximately 200 amino acids structured in two subdomains of 100 amino acids (fibronectin type III modules), themselves structured by seven β-strands arranged in a β-sandwich. The 200 amino-acids domain usually contain the ligand binding site. IFNAR2, IFNLR1, IL10R2, IFNGR1 and IFNGR2 are classical representatives of this family, while IFNAR1 is atypical as its extracellular domain is duplicated. GAS, IFN-γ-activated site; IRF9, IFN regulatory factor 9; ISGF3, IFN-stimulated gene factor 3, refers to the STAT1–STAT2–IRF9 complex; ISRE, IFN-stimulated response element; P, phosphate; STAT1/2, signal transducers and activators of transcription 1/2.
