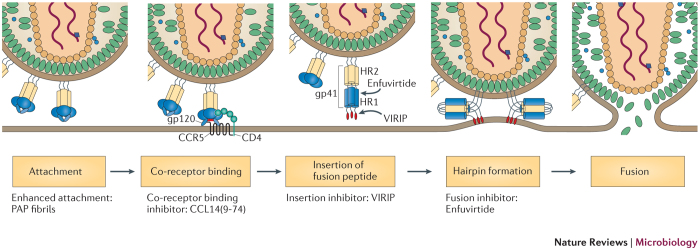
Figure 2. Endogenous peptides that affect HIV-1 infection.
Overview of the HIV-1 entry steps that are targeted by peptides. Amyloidogenic peptides that are derived from human semen, such as fragments of prostatic acidic phosphatase (PAP), can self-assemble into amyloid fibrils that promote the attachment of the virus to target cells by neutralizing the repulsion between negatively charged viral and cellular membranes8,61. Unlike these semen-derived enhancing agents, human peptides that are isolated from haemofiltrate have antiviral activity. The truncated form of the abundant human chemokine CC-chemokine ligand 14 (CCL14(9–74)) inhibits entry of R5-tropic HIV-1 strains by binding to the CC-chemokine receptor 5 (CCR5) co-receptor6,42. Human-derived virus-inhibitory peptide (VIRIP; which is a subfragment of the serine protease inhibitor α1-antitrypsin) also targets the fusion peptide by specifically interacting with the amino terminus of its transmembrane domain to block penetration and insertion of the viral fusion peptide8. Enfuvirtide is a synthetic peptide that is derived from an α-helical region in the HIV-1 transmembrane glycoprotein36. This inhibitor binds to helical repeat region 1 (HR1) of gp41, which is a region that is distinct from the region that is targeted by VIRIP, and prevents the formation of a hairpin structure that is required for membrane fusion, thereby blocking HIV-1 infection. The target sites for VIRIP and enfuvirtide in gp41 are indicated. From Forssman, W.-G. et al. Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide. Sci. Transl. Med. 2, 63re3 (2010). Reprinted with permission from AAAS.