Figure 2. Protein sequences of testis ACE and the N- and C-domains of somatic ACE.
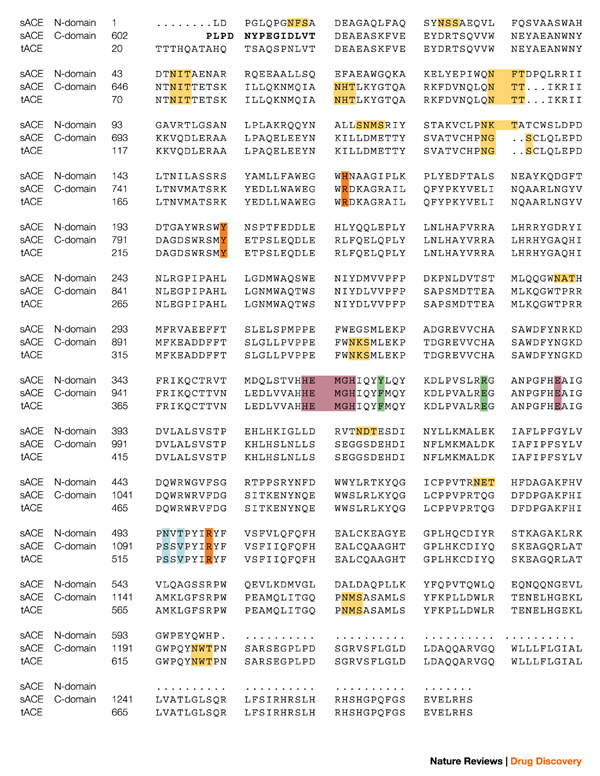
The sequences are aligned and numbered according to Refs 18,26. The bridge region is in bold and the zinc-binding motif, as well as the third zinc ligand (Glu), are in purple. The C-domain N-glycosylation sites are in yellow, the chloride ligands in orange, and the active site residues depicted in Fig. 4 are in blue and green. The secondary structure elements for tACE5 structure α-helices (α), β-strands (β) and 310 helices are: α1(40–71); α2(74–100); H1(101–107); α3(109–120); H2(122–127); α4(128–149); β1(150–153); β2(157–161); α5(163–172); α6(174–211); α7(215–222); H3(223–225); α8(228–260); β3(270–272); H4(283–285); α9(286–291); α10(300–308); α11(311–326); α12(332–339); β4(355–359); β5(364–368); α13(374–394); H5(398–402); α14(406–430); α15(439–473); α16(480–494); β6(495–496); H6(506–511); α17(520–541); H7(546–550); α18(555–568); α19(573–583); α20(589–610). sACE, somatic angiotensin-converting enzyme; tACE, testis angiotensin-converting enzyme.
