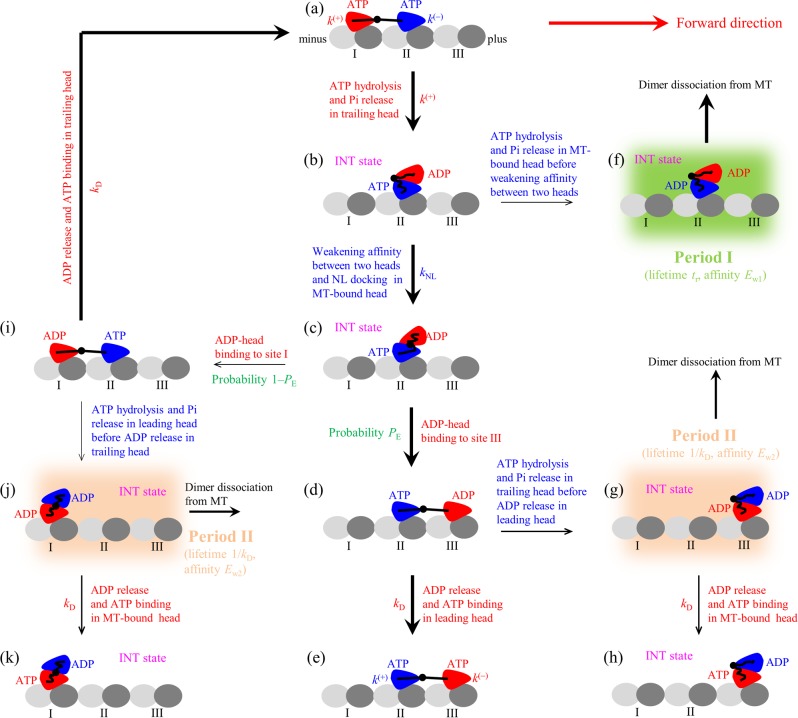
Figure 1.
Model of kinesin chemomechanical coupling. (a–k) Pathway of kinesin stepping at saturating ATP and occurrence of weak MT-binding periods (including periods I and II) when the dimer binds weakly to MT (see text for detailed descriptions). The thickness of the arrow denotes the magnitude of the transition rate or probability under no load. For simplicity, ATP hydrolysis and Pi release are treated here as one step with the symbol ATP representing both ATP and ADP·Pi states, because in both ATP and ADP·Pi states the head binds strongly to the MT. As a result, the change of ATP to ADP shown here consists of two sequential transitions including the transition of ATP to ADP·Pi and that of ADP·Pi to ADP. Since at saturating ATP after ATP release from the leading head, another ATP molecule can bind immediately, and the lifetime of the nucleotide-free state is so short that the nucleotide-free state is neglected here.