Figure 2.
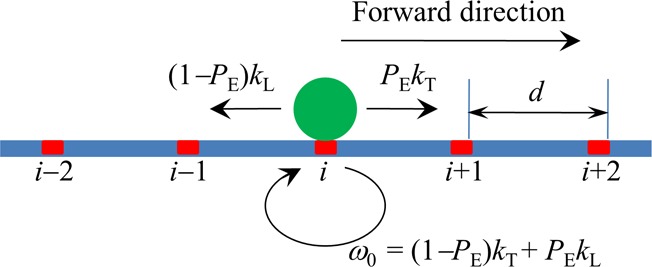
Simplified model of stepping of the kinesin dimer at saturating ATP. The simplified model is derived from the pathway illustrated in Figure 1 where the two weak MT-binding periods (including periods I and II) that can only occur with very low probabilities in a chemomechanical coupling cycle can be neglected. The green circle denotes the kinesin dimer. The binding sites on the MT filament are indicated by ..., (i – 1), i, (i + 1), .... The kinesin dimer steps forward with the rate PEkT and backward with the rate (1 – PE)kL where PE is the effective chemomechanical coupling probability, kT is the ATPase rate of the trailing head, and kL is the ATPase rate of the leading head.
