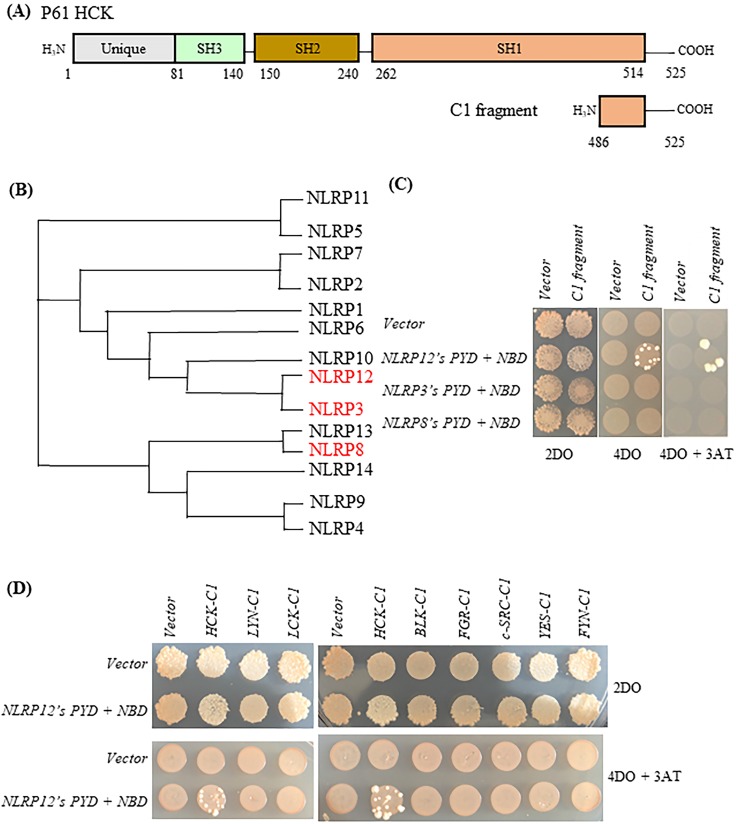
Figure 2.
Selectivity of binding of NLRP12 with HCK. (A) Schematic drawing of HCK (p61 isoform, top) and, for comparison, the region of HCK (“HCK C1 fragment,” lower) used for testing the selectivity of binding of HCK to other members of the NLRP family of proteins. The numbers represent the amino acid residues of the protein or protein fragment. (B) Phylogenetic tree showing the relationships among the PYD + NBD region of all of the members of the human NLRP family of proteins. The phylogenetic tree was produced through Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). For each NLRP family member, the PYD + NBD of the longest isoform was selected for generating the phylogenetic tree. NLRP3 is phylogenetically the closest NLRP to NLRP12, while NLRP8 is phylogenetically among the most distant from NLRP12. NLRP3 and NLRP8 are highlighted in red color. (C) When NLRP12's PYD + NBD and HCK C1 fragment were co-transformed into yeast, they grew on the highest stringency plates (4DO + 3AT), consistent with an interaction between the two constructs. On the other hand, neither NLRP3's PYD + NBD co-transformed with the C1 fragment nor NLRP8's PYD + NBD co-transformed the C1 fragment grew on the highest stringency plates. These results are consistent with a selective interaction between NLRP12 and the HCK C1 fragment, but not with the other NLRPs. (D) Yeast clones grew on the high stringency plates 4DO + 3AT when HCK's C1 fragment and NLRP12's PYD + NBD were co-transformed, but they did not grow on the high stringency 4DO + 3AT plates when the C1 fragments of other members of the c-SRC family of non-receptor tyrosine kinases were co-transformed with NLRP12's PYD + NBD. These results are again consistent with a selective interaction between NLRP12 and HCK.