Figure 4. Molecular interactions of carbohydrate-binding agents (CBAs) with carbohydrate oligomers.
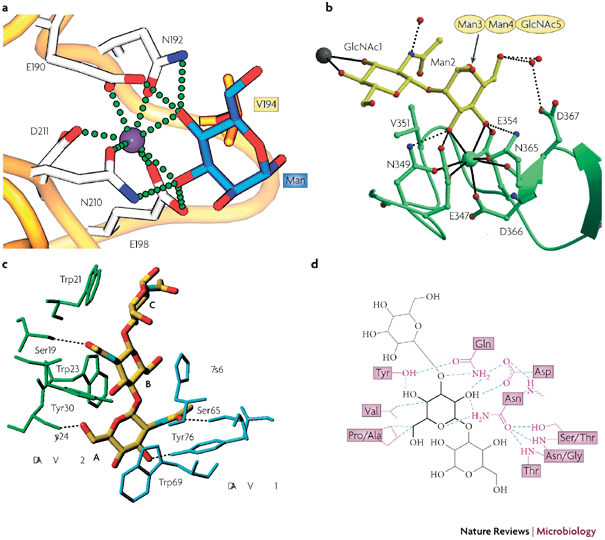
a | The terminal mannose (Man) of Manα1–3Man is complexed to liver MBP–C64 (Protein Data Bank (PDB) ID 1kza). Calcium is depicted as a purple sphere, with coordinating residues in stick representation. The amino-acid residue Val194 (in orange) participates in van der Waals interactions. The calcium coordination and hydrogen bonds between protein and carbohydrate (blue) are shown as green spheres. b | Ribbon diagram of dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing non-integrin (DC-SIGN) complexed with Man3 −(N-acetylglucosamine)2 (Man3GlcNAc2)65 (PDB ID 1k9i). Calcium is shown as a green sphere, whereas the carbohydrate is shown in a yellow stick representation. The calcium coordination is shown in detail, with the hydrogen bonds to important coordinating residues shown as dotted spheres. The gray sphere represents a calcium site of another carbohydrate-recognition domain. c | Hydrogen bonding, van der Waals interactions and aromatic ring stacking of GlcNAc3, with the GlcNAc-specific lectin from Urtica dioica (UDA)37 (PDB ID 1ehh). Two isolectin VI molecules are shown in blue and green. Dotted lines are hydrogen-bonding contacts between UDA and GlcNAc3. Sugar residues are labelled with A, B, and C from the non-reducing end. d | Hydrogen-bonding pattern and van der Waals contacts of the Narcissus psuedonarcissus lectin 7 (NPL7) carbohydrate-binding domain complexed to Manα(1– 3)Man66 (PDB ID 1npl). The mannose residue in the main binding pocket is shown in bold lines, with the hydrogen bonds depicted as dotted lines and van der Waals contacts as purple lines. MBP-C, mannose-binding protein C. Panel a reproduced with permission from Ref. 64 © (2005) Elsevier Science. Panel b reproduced with permission from Ref. 65 © (2001) American Association for the Advancement of Science. Panel c reproduced with permission from Ref. 37 © (2000) Elsevier Science. Panel d reproduced with permission from Ref. 66 © (1999) Elsevier Science.
