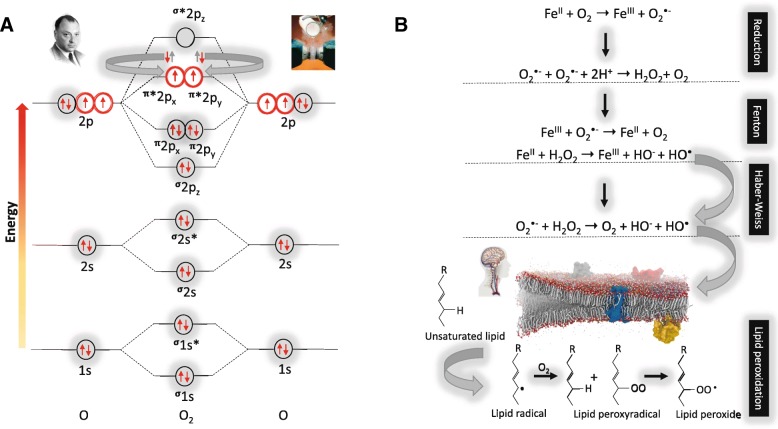
Fig. 3.
a Molecular orbital diagram of the most stable form (electronic ground state) of the diatomic oxygen molecule (3∑g−O2) and b. Biological reactions underpinning oxygen toxicity. a Each line represents a molecular orbital and the arrows represent electrons, the direction of which indicates their spin quantum number. Note that oxygen (O2) with an electronic structure of 1s22s22p4 qualifies as a di-radical since it contains two unpaired electrons each occupying different π*2p anti-bonding orbitals (highlighted in red) with the same spin quantum number (parallel spin) in accordance with Hund’s rule. It is for this reason that O2 is paramagnetic allowing liquid O2 to hang magically suspended between the poles of a magnet (upper right insert). During the process of oxidation when O2 looks to accept a (spin opposed) pair of electrons (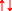 ), only one of the pair (
), only one of the pair ( ) can “fit” into each of the vacant π*2p anti-bonding orbitals to create a spin opposed pair (as indicated). Hence, O2 thermodynamically prefers to accept only one electron at a time to conform with the Pauli Exclusion Principle [named after the Nobel Prize winning work of the Austrian physicist Wolfgang Pauli (1900–1958), photograph upper left insert]. Fortuitously, this “spin restriction” means that O2 reacts “sluggishly” with the brain’s organic compounds with the organic donor having to undergo a “slow spin inversion’” to donate its electrons. b Three types of reactions lead to the superoxide anion (O2•−)-mediated formation of the damaging hydroxyl radical (HO•) capable of causing indiscriminate damage to biological cell membranes that characterizes O2 toxicity; [1] one-electron reduction of molecular O2 to O2•− catalyzed by transition metals including iron (Fe), [2] Fenton reactions that involve metal-catalyzed formation of HO• and [3] Haber-Weiss reaction involving the combination of O2•− and hydrogen peroxide (H2O2) to yield additional HO•
) can “fit” into each of the vacant π*2p anti-bonding orbitals to create a spin opposed pair (as indicated). Hence, O2 thermodynamically prefers to accept only one electron at a time to conform with the Pauli Exclusion Principle [named after the Nobel Prize winning work of the Austrian physicist Wolfgang Pauli (1900–1958), photograph upper left insert]. Fortuitously, this “spin restriction” means that O2 reacts “sluggishly” with the brain’s organic compounds with the organic donor having to undergo a “slow spin inversion’” to donate its electrons. b Three types of reactions lead to the superoxide anion (O2•−)-mediated formation of the damaging hydroxyl radical (HO•) capable of causing indiscriminate damage to biological cell membranes that characterizes O2 toxicity; [1] one-electron reduction of molecular O2 to O2•− catalyzed by transition metals including iron (Fe), [2] Fenton reactions that involve metal-catalyzed formation of HO• and [3] Haber-Weiss reaction involving the combination of O2•− and hydrogen peroxide (H2O2) to yield additional HO•