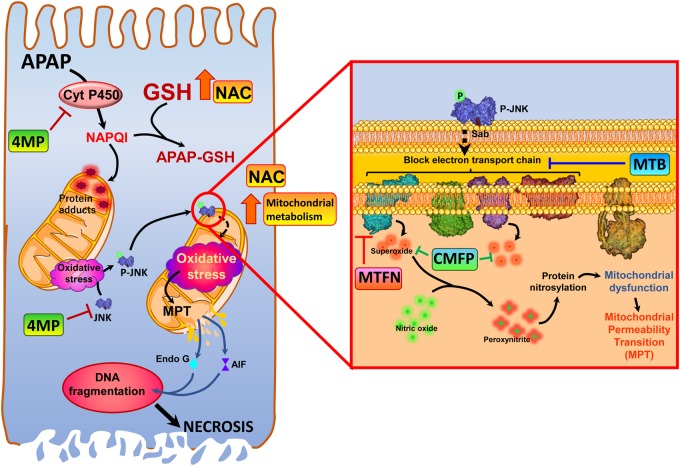
Figure 1.
Pharmacological targets for acetaminophen hepatotoxicity. Acetaminophen (APAP, N-acetyl-p-aminophenol, paracetamol)-induced liver injury is initiated by its metabolism by cytochrome P450 enzymes to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which depletes cellular glutathione and forms protein adducts, especially on the mitochondria. This results in mitochondrial oxidative stress, which results in activation of the mitogen-activated protein c-Jun N-terminal kinase (JNK), and its translocation to the mitochondria. On the mitochondria (inset) phosphorylated JNK binds to the outer membrane protein Sab, and initiates a blockade of the electron transport chain, which subsequently induces release of superoxide from respiratory complexes I and III. Superoxide reacts with nitric oxide within mitochondria to form the highly reactive nitrogen species peroxynitrite, which results in protein modification by nitration of tyrosine residues. This amplifies the mitochondrial dysfunction, ultimately causing induction of the mitochondrial permeability transition and release of mitochondrial proteins such as endonuclease G and apoptosis inducing factor into the cytosol. Nuclear translocation of these proteins then induces DNA fragmentation, and finally hepatocyte necrosis. The standard of care antidote N-acetylcysteine protects against APAP hepatotoxicity by increasing GSH resynthesis, thus replenishing GSH stores, and also facilitating mitochondrial energy metabolism to surmount detrimental effects of protein adduct formation. Newer therapeutic interventions have alternate mechanisms of action, with the majority targeting mitochondrial dysfunction, while 4-methylpyrazole acts upstream, inhibiting cytochrome P450-mediated NAPQI formation as well as inhibiting JNK activation. Calmangafodipir functions as a superoxide dismutase mimetic, scavenging superoxide to prevent formation of peroxynitrite, whereas metformin inhibits mitochondrial oxidant stress by inhibition of respiratory complex I. Methylene blue, however, improves mitochondrial function by bypassing electron transport chain blockade and preventing formation of free radicals.