Figure 5.
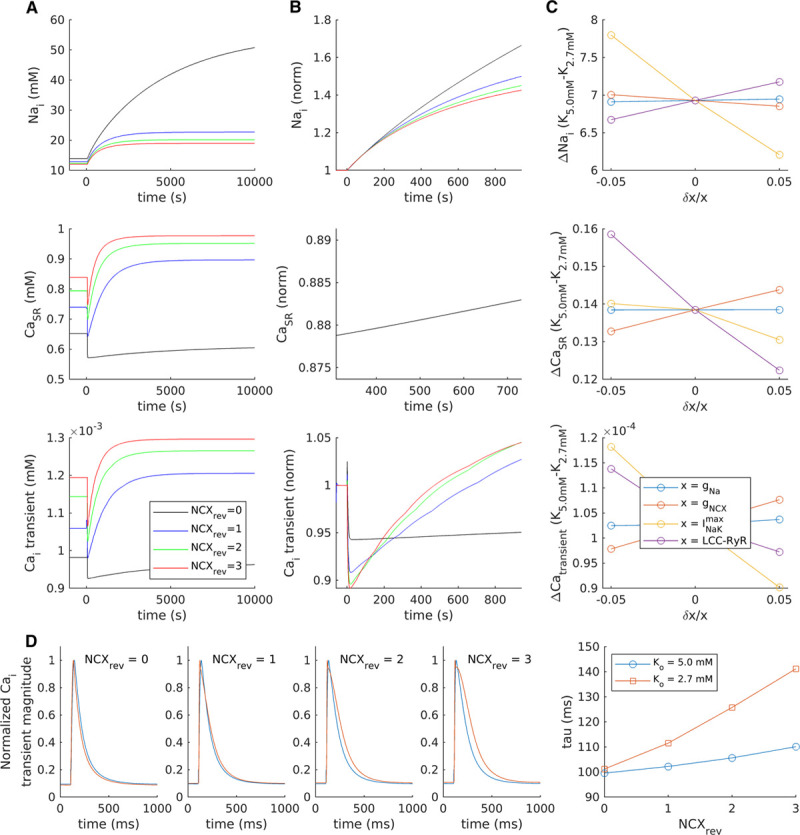
Close Na+, K+-ATPase (NKA)-Na+-Ca2+ exchanger (NCX) crosstalk drives the biphasic Ca2+ transient response to hypokalemia. A mathematical model of the rat ventricular cardiomyocyte was employed to investigate the contribution of various ion channels and transporters to changes in cellular [Na+], SR Ca2+ content, and Ca2+ transients. A, With baseline model settings (blue lines), reducing [K+]o from 5.0 to 2.7 mmol/L triggered a modest accumulation of intracellular [Na+], and a biphasic response of SR Ca2+ content and Ca2+ transients which occurred over a markedly longer time course than that observed experimentally. To simulate greater crosstalk between NCX and NKA, the fraction of NCX operating in reverse mode (NCXrev) was increased in a step-wise manner, which produced an augmented and accelerated biphasic response (see also enlargement of the first 1000 s in [B], presented normalized to [K+]o=5.0). Removing the NCXrev contribution prevented any secondary rise in Ca2+ transients, in resemblance to experiments in untubulated cells. C, Sensitivity analyses were performed to examine the effects of modulating individual ion fluxes±5%. Reducing t-tubule-associated fluxes such as INa (gNa) or the number of L-type-RyR units (LCC-RYR) did not inhibit the biphasic response, while changing NCX and NKA fluxes had proportionally opposite effects. D, In addition to augmenting the biphasic response to hypokalemia, increasing NCXrev slowed Ca2+ transient decay at steady state.
