Abstract
Polyploidization is a widespread mechanism of evolutionary divergence in flowering plants. Ecological divergence of polyploid lineages has been proposed as a key process shaping the distribution of cytotypes in nature (niche shift hypothesis); however, evidence for the role of niche separation in replicated diploid-polyploid species pairs is still needed. This study aimed to assess the role of abiotic factors shaping current cytotype distributions. For that, we examined the distribution and environmental niches of two varieties recognized in diploid-tetraploid Jasione maritima across the species range and within a putative contact zone on the Iberian Peninsula. We counted chromosomes, screened for ploidy across Iberian Peninsula and characterized environmental requirements using niche modeling tools. We found that J. maritima var. maritima is composed by diploids with disjunct distribution in the west coast of France and northwest Iberian Peninsula, and by tetraploids in Iberian Peninsula, while var. sabularia is tetraploid. In the Iberian Peninsula, two parapatric contact zones along a linear coastal distribution were detected, one between diploid and tetraploid var. maritima, and the other between tetraploids of the two varieties. Environmental variables of diploid populations from France are distinct from those of southern diploid populations, which are more similar to tetraploids. In general, niche modeling results are congruent with the observed distribution patterns, although the results suggest a wider contact zone between varieties and cytotypes. Tetraploids of both varieties revealed different degrees of environmental divergence in comparison with their diploid counterpart. Tetraploid var. sabularia differed environmentally from diploids suggesting niche divergence. In contrast, tetraploid var. maritima overlapped with diploid environmental niche and currently occupies its entire predicted range, whereas diploids are restricted to northern areas of their suitable environment. Differences in ecological envelopes facilitate the recognition of functional units of biodiversity within polyploid groups, allowing the study of factors related to post-polyploidization divergence. Thus, whereas changes in environmental requirements may have allowed tetraploid var. sabularia to spread in habitats not favorable to diploids, other factors are involved with the distribution of diploid and tetraploid var. maritima.
Keywords: parapatric distribution, cryptic diversity, diploids, Jasione maritima, niche modeling, tetraploids
Introduction
Whole genome duplication leading to polyploidy is a widespread mechanism of plant evolution and diversification (Soltis and Soltis, 1999; Soltis et al., 2010; Jiao et al., 2011). Estimates of the incidence of polyploidy in angiosperms range from 20 to 40% (e.g., Stebbins, 1938, 1950; Wood et al., 2009) and is considerably higher in specific geographical regions (e.g., 69–87% in the Arctic Flora, Brochmann et al., 2004; 37–49% in the Mediterranean Basin, Marques et al., 2017). Most studies of the incidence of polyploidy are based on chromosome counts obtained during taxonomic studies; however, due to technical and logistical difficulties, such studies are usually based on a few counts per species, which can limit one’s ability to detect multiple cytotypes (Bennett, 1998; Soltis et al., 2007; Marques et al., 2017). With the emergence of high throughput methods such as flow cytometry, the number of studies focused on the incidence of polyploidy has increased (Kron et al., 2007; Husband et al., 2013) and revealed that the extent of polyploidy is underestimated in many plant species (Marques et al., 2017).
Flow cytometry has enabled detailed studies of polyploid complexes and has thus provided novel insights into geographic patterns of cytotype diversity, from within populations to across the entire species geographic range (e.g., Aster amellus, Castro et al., 2012; Chamerion angustifolium, Husband and Schemske, 1998; Erysimum mediohispanicum, Muñoz-Pajares et al., 2018; Knautia arvensis agg., Kolář et al., 2009; Mercurialis annua, Buggs and Pannell, 2007; Ranunculus adoneus, Baack, 2004). These large-scale studies of diploid-polyploid complexes have revealed a wide variety of cytotype compositions and geographic distributions, confirming that polyploidy is a complex and dynamic process in nature. According to Petit et al. (1999), the majority of cytotypes in polyploid complexes grow in close proximity, at least in part of their distribution range, and form parapatric or sympatric contact zones (e.g., Kolář et al., 2009; Castro et al., 2012; Muñoz-Pajares et al., 2018). Cytotype distributions are the result of multiple processes, including the evolutionary history of the complex, frequency of polyploid formation, divergent abiotic tolerances and biotic interactions (e.g., competition, herbivores), as well as dispersal abilities and inter-cytotype breeding barriers (Petit et al., 1999; Levin, 2002; Lexer and van Loo, 2006). While cytotypes in most polyploids have complex distributions, it is rarely known which process or combination of processes account for such complexity.
Abiotic factors, such as temperature and precipitation, play a key role in shaping distributions of species and biodiversity. Because polyploidization creates novelty, it may drive direct or indirect changes in phenotypes and result in shifts in ecological tolerances (Levin, 1975; Husband and Schemske, 2000; Maherali et al., 2009; Ramsey, 2011; Manzaneda et al., 2012, 2015; Hao et al., 2013). One of the immediate effects of polyploidization is the increase in cell size (Stebbins, 1971; Masterson, 1994), which is related to changes in physiological traits. For example, genome duplication frequently leads to increased stomata size and lower stomata densities (Li et al., 1996; Maherali et al., 2009), thicker epidermis (Li et al., 1996), or changes in xylem structure, cell size and wall thickness, and lignin content (Nassar et al., 2008; Maherali et al., 2009; Hao et al., 2013). These changes can affect gas exchange and water transport and generate trade-offs between water transport efficiency and cavitation risk, and often leads to greater drought tolerance in polyploids than their diploid relatives (e.g., Maherali et al., 2009; Manzaneda et al., 2012, 2015; Hao et al., 2013). Such physiological differences acquired by polyploids may have significant ecological implications (Ramsey and Schemske, 2002), allowing ecological niche expansion of the polyploid lineages (niche shift hypothesis; Levin, 1975; Fowler and Levin, 1984; Husband and Schemske, 2000; Parisod and Broennimann, 2016). Niche differentiation between polyploids and their diploid relatives may thus enable polyploids to expand to areas unoccupied by diploids and avoid direct competition (Levin, 1975, 2003; Husband and Schemske, 2000); however, evidence for the role of environment in accounting for distribution differences between diploids and polyploids remains largely unexplored.
Niche modeling (e.g., Ecological Niche Modeling; Warren et al., 2008, 2010) and multivariate analyses of predictor variables (Broennimann et al., 2012) are useful tools for examining relationships between large-scale cytotype distributions and environmental tolerances. These methods take advantage of global climate and habitat databases to relate the geographic distributions of diploid and polyploid populations to spatial environmental data, supporting the creation of predictions about niche shifts or niche conservation associated with whole genome duplication (e.g., Glennon et al., 2012; Godsoe et al., 2013; Thompson et al., 2014; Visger et al., 2016; Muñoz-Pajares et al., 2018; López-Jurado et al., 2019; Molina-Henao and Hopkins, 2019). Such approaches are powerful in scope and useful for generating hypotheses that can be further tested using manipulative experiments (Glennon et al., 2014; Marchant et al., 2016), such as reciprocal transplants (e.g., C. angustifolium, Martin and Husband, 2013).
The genus Jasione L. (Campanulaceae) comprises 16 species found mostly in the Mediterranean region (Pérez-Espona et al., 2005). It is a relatively old genus (Bokhari and Sales, 2001; Haberle et al., 2009) and diversification appears to have occurred recently, possibly during the last glaciation cycle in Europe (Bokhari and Sales, 2001; Pérez-Espona et al., 2005). The Iberian Peninsula is the center of morphological diversity in the genus, with variation among the 10 accepted species (Sales and Hedge, 2001) in life history, ploidy and habitat (Bokhari and Sales, 2001; Sales and Hedge, 2001, Rubido-Bará et al., 2010). Jasione maritima (Duby) Merino comprises two varieties: J. maritima var. maritima occurring in western France and in northwestern Spain, and J. maritima var. sabularia (Cout.) Sales and Hedge from the north coast of Portugal (Sales and Hedge, 2001). The species also has been cited for one locality in the coast of the Basque Country, near Bilbao (Guinea, 1949), but it has been considered doubtful and the species has not been located again in this region (Aseginolaza et al., 1984). The var. maritima in France was identified as diploid (2n = 2x = 12 chromosomes; Delay, 1967), whereas plants in Spain are tetraploid (2n = 4x = 24 chromosomes; Lago Canzobre and Castroviejo, 1992; Rubido-Bará et al., 2010); var. sabularia has been described as strictly tetraploid (Leitão and Paiva, 1988). However, a recent study reported the presence of two genome size categories in some Galician populations of var. maritima, namely 2C = 3.44 ± 0.04 pg in the northern locations and 2C = 6.62 ± 0.23 pg in the southern ones (Rubido-Bará et al., 2010). This may suggest the presence of ploidy variation on the Iberian Peninsula. The distribution of diploids in northern locations and tetraploids in southern ones may suggest niche differentiation between cytotypes, making this system ideal to explore the role of environmental variables in niche separation.
In this study we explored the role of niche divergence driving current cytotype and varieties distribution patterns in J. maritima complex. For that, we (1) investigate the diversity of cytotypes throughout the range of J. maritima, (2) describe the frequency and distribution of cytotypes within and among natural populations, and (3) examine the correspondence between environmental variables and cytogeographic patterns in the species. We quantified ploidy variation using chromosome counts and flow cytometry over the species’ distribution in the Iberian Peninsula, a putative contact zone between the reported cytotypes (from northwestern to central coast). We then used niche modeling tools and multivariate analyses to determine whether the observed cytotype distribution patterns could be explained by environmental variables. The environmental analyses were performed at two spatial scales: (1) the entire distribution range (1 km resolution) and (2) the northwestern coast of the Iberian Peninsula (100 m resolution). We tested the hypothesis that cytotype distribution in J. maritima reflects the ecological divergence between diploids and their polyploid descendants.
Materials and Methods
Study System and Field Sampling
Jasione maritima (Duby) Merino is a perennial herb that grows in sand dune systems from Ferrol (Galicia, Spain) to São Jacinto (Aveiro, Portugal) in the Iberian Peninsula, and in the west coast of France (Sales and Hedge, 2001). The plant forms a leafy rosette in winter and produces capituliform inflorescences in the summer. The inflorescence is composed of blue to lilac, insect pollinated flowers, each producing dozens of small seeds that germinate from autumn to late winter (Sales and Hedge, 2001; M. Castro, field observations).
Field surveys were conducted during the flowering season (June and July), from 2013 to 2015, within and beyond the known distribution limits of J. maritima in the Iberian Peninsula. All locations visited were geo-referenced (see Supplementary Table 1 for the 35 J. maritima populations). Plants in each population were identified based on indumentum and leaf shape following Flora Iberica (Sales and Hedge, 2001) as J. maritima var. maritima or J. maritima var. sabularia. Within each population, we randomly sampled 5–6 fresh leaves from each of 4–30 individuals and stored them in hermetic plastic bags at 4–8°C to assess genome size and DNA ploidy using flow cytometry. For chromosome counts, seeds from up to 15 plants were collected in paper bags from selected locations, based on preliminary genome size estimates and including one population of each genome size category (Supplementary Table 1). Herbarium specimens were collected, and vouchers deposited in SANT herbarium (Supplementary Table 1).
Chromosome Counts
Chromosome counts were attained following the protocol of Goldblatt and Takei (1993) with some modifications. Briefly, seeds from one population per genome size category (Supplementary Table 1) were germinated and grown in 1L pots with commercial soil in an experimental garden. Actively growing root tips were harvested and pre-treated in 0.002 M aqueous 8-hydroxyquinoline at room temperature for 4 h and 30 min, after which root tips were fixed in a solution of 3:1 of 96% ethanol and glacial acetic acid for at least 24 h at 4°C. Roots tips were then hydrolyzed in 1 N HCl at 60°C in a sand bath for 5 min, submerged in Schiff reagent (based in Greilhuber and Ebert, 1994) for 1 h and 30 min, washed in Sulfur water three times for 10 min periods, and finally squashed under a glass cover in aseptic orcein 2%. Chromosome spreads were observed using a Nikon Eclipse 80i light microscope and photographed using a Nikon Plan Apo VC 100 × /1.40 oil-immersion lens, with a Q Imaging Retiga 2000R Fast 1394 digital camera and Q-Capture Pro v.7 software. Based on the 2n chromosome counts, plants were assigned to a ploidy level (diploid for 2n = 12, or tetraploid for 2n = 24). We used these same plants as reference material to determine DNA ploidy of each genome size category and estimate the DNA ploidy of the remaining individuals using flow cytometry.
Genome Size and DNA Ploidy Estimates
We measured genome size and DNA ploidy in all plants using flow cytometry. Following Galbraith et al. (1983), 50 mg of leaf material of each sample was chopped with 50 mg of leaves of an internal reference standard (Solanum lycopersicum “Stupické”, hereafter S.l., with 2C = 1.96 pg; Doležel et al., 1992, 2007) using a sharp razor blade in a glass Petri dish with 1 ml of WPB buffer (0.2 M Tris–HCl, 4 mM MgCl2.6H2O, 1% Triton X-100, 2 mM EDTA Na2.2H2O, 86 mM NaCl, 10 mM metabisulfite, 1% PVP-10, pH adjusted to 7.5 and stored at 4–8°C; Loureiro et al., 2007). The nuclear suspension was filtered through a 50 μm nylon filter to remove coarse debris and 50 μg ml–1 propidium iodide (PI; Fluka, Buchs, Switzerland) and 50 μg ml–1 RNAse (Fluka) were added to stain the DNA and avoid staining dsRNA, respectively. After 5 min of incubation, the samples were analyzed in a Partec CyFlow Space flow cytometer (532 nm green solid-state laser, operating at 30 mW; Partec GmbH., Görlitz, Germany). The results were visualized using Partec FloMax software v2.4d (Partec GmbH, Münster, Germany) in four graphics: histogram of fluorescence pulse integral in linear scale (FL); forward light scatter (FS) vs. side light scatter (SS), both in logarithmic (log) scale; FL vs. time; and FL vs. SS in log scale. To remove debris, a polygonal region was defined in the FL vs. SS histogram and subsequently applied to all graphics. At least 1300 nuclei from sample and standard G1 peaks were analyzed per sample (Suda et al., 2007). Only samples with a coefficient of variation (CV) of 2C peak <5% were included; when a sample failed to meet this standard, it was re-prepared and analyzed until such quality was achieved (Greilhuber et al., 2007). In 15 populations (six diploid var. maritima, six tetraploid var. maritima and three tetraploid var. sabularia), 2–10 individuals were analyzed individually for genome size. For the remaining individuals and populations, the samples were pooled (5–6 individuals plus the reference standard) providing estimates of DNA ploidy only. The holoploid genome size (2C in pg; sensu Greilhuber et al., 2005) was calculated using the formula:
DNA ploidy was inferred for each sample based on the chromosome counts and genome size estimates obtained for the selected populations. The monoploid genome size (1Cx; sensu Greilhuber et al., 2005; mass in pg) was calculated by dividing the holoploid genome size (2C) by the assigned DNA ploidy. Based on plant identification and ploidy level estimates, each population was classified as follows: diploid J. maritima var. maritima (2x var. maritima), tetraploid J. maritima var. maritima (4x var. maritima) or tetraploid J. maritima var. sabularia (4x var. sabularia).
Descriptive statistics of 2C genome size were calculated for each cytotype (mean, SD, CV, and range) based on individual flow cytometric estimates, only. Mean and SD were also calculated for the 1Cx genome size. To assess differences between diploids and tetraploids in 2C and 1Cx genome sizes, we used generalized linear models (GLM) (Bolker et al., 2009) with a Gaussian distribution and an identity link function. Cytotype was treated as a fixed factor and genome size as the response variable. Statistical analyses were performed in R software version 3.0.1 (R Core Development Team, 2016), using the packages “car” for Type-III analysis of variance (Fox et al., 2015), “lme4” for generalized linear models (GLM; Bates et al., 2014) and “multcomp” for multiple comparisons after Type-III analysis of variance (Hothorn et al., 2017).
Environmental Niche Modeling
Environmental niches of the two varieties of J. maritima and the cytotypes within var. maritima (i.e., 2x var. maritima, 4x var. maritima and 4x var. sabularia) were compared using GLM analyses and niche modeling tools. We conducted these analyses at two spatial scales: (1) entire geographic distribution of J. maritima (in Portugal, Spain, and France); and (2) the putative contact zone (in the northwest Iberian Peninsula).
The presence database was generated based on our field observations in the Iberian Peninsula, herbarium specimens from COI and SANT (acronyms following Thiers, 2017), literature survey of karyologic reports with geographical information, and GBIF database.1 For France, only karyologic reports and GBIF information were used. All reports from France were classified as diploid, based on previous chromosome counts (Delay, 1967). The final database included 40 records for diploid var. maritima, 21 records for tetraploid var. maritima and 18 records for tetraploid var. sabularia.
To examine environmental niches at the scale of the entire species range, 19 bioclimatic variables (Bio1-Bio19) plus elevation were extracted for all species occurrences from the Worldclim database2 at a resolution of 30 arc-seconds (approx. 1 km; Supplementary Table 2), using R package “dismo” (Hijmans et al., 2017). At the scale of the putative contact zone, the following set of variables were used at 100 m resolution to account for the restricted habitat of the species: elevation;3 topographic variables including aspect (in degrees: 310–360° and 0–45° exposed to N – cooler; 45–135° exposed to E; 135–220° exposed to S – hotter; 220–310° exposed to W – humid), slope, topographic position index (TPI; mm) and incoming solar radiation for the summer period;4 climatic variables including summer mean temperature (June to August) and mean annual precipitation;5 lithology;6 and distance from the sea (Supplementary Table 3). Additionally, latitude and longitude were also included in both approaches (Supplementary Tables 2, 3). Different variables were used in the two approaches because of differences in the available parameters at the two resolution scales.
Differences in extracted variables among cytotypes were evaluated using GLMs, with cytotype as a fixed factor and each variable as a response variable. A Gaussian distribution with an identity link function was used for continuous variables and a Poisson distribution with a log link function was used for discrete variables. Exploratory principal component analyses (PCA) were also performed to evaluate the contribution of each variable for the total variance. Correlations between the variables were obtained using Pearson/Spearman coefficients. For each set of variables with correlation values higher than 0.7, only one was selected. Taking into account the results of the correlations and the PCAs (Supplementary Tables 2–4 and Figure 1), a set of non-correlated variables were selected for niche modeling analyses: in the entire range approach, mean diurnal range, isothermality, temperature seasonality, precipitation of the wettest month and precipitation seasonality (Supplementary Tables 2, 4) were selected; for the putative contact zone approach, slope, summer mean temperature, mean annual precipitation and distance to coast (Supplementary Tables 3, 4) were used.
Niche modeling analyses were performed with maximum entropy modeling (MaxEnt) using the R software package “biomod2” (Thuiller et al., 2016). Spatial predictive models were calibrated based on the selected variables and on presence/absence data. Absences were derived from 19 confirmed observations from field surveys, occurrence records for the other two cytotypes (namely, in the 2x var. maritima dataset, 4x var. maritima and 4x var. sabularia populations were considered as absences, and vice-versa in the 4x var. maritima and 4x var. sabularia datasets) and 5000 pseudo-absences. For pseudo-absences, a buffer around each presence point (of 3 km in the entire range approach and 300 m in the contact zone approach) was applied and 5000 points were randomly selected within the remaining area (defined as background points). To reduce uncertainty and to produce robust models, each technique was replicated 30 times using random subsets obtained from each dataset. The presence database of each entity was divided randomly into training (70%) and test (30%) subsets (Phillips et al., 2006; Araújo and New, 2007). All subsets were statistically independent, since in each subset, each occurrence was used only once, as training or as test occurrence (Phillips, 2008). Models were evaluated based on the independent accuracy measure AUC of ROC (area under the curve of the receiver operating characteristic), and only models with AUC > 0.7 were combined to produce the final model for each entity (Supplementary Table 5). The final models were converted to a binary format (using the default threshold of 0.5) and were used to calculate niche overlap between the entities.
The percentage of niche overlap was quantified for the three paired cytotype comparisons (i.e., 2x var. maritima vs. 4x var. maritima; 2x var. maritima vs. 4x var. sabularia; and 4x var. maritima vs. 4x var. sabularia) at the whole range and putative contact zone scales. Niches were compared through an ordination approach using a PCA calibrated with environmental values (Di Cola et al., 2017) using “ecospat” (Broennimann et al., 2012) and “raster” (Hijmans et al., 2017) R packages. The PCA calculates the occurrence density and environmental factor density along environmental (principal component) axes for each pixel, maximizing the ecological variance of the areas of the cytotypes. Then, PCA scores of the cytotype distributions were projected onto a grid of cells bounded by the maximum and minimum PCA scores; this allows the visual assessment of the overlap and dynamics of the environmental niches of the cytotypes being compared (Di Cola et al., 2017). All models and analyses were performed in the R 3.3.0 environment (R Core Development Team, 2016).
Results
Cytogenetic Diversity
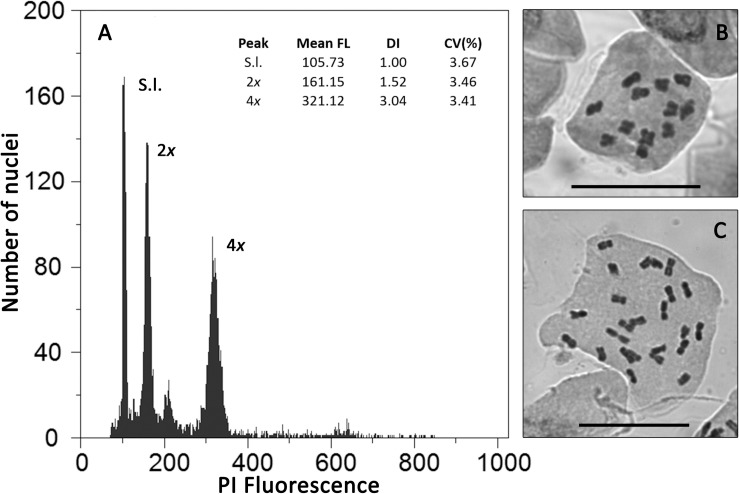
Flow cytometric histograms were of good quality, with all samples used to assess genome size having CV values below 5% (Figure 1A and Supplementary Table 6). Each genome size category corresponded to different chromosome numbers (Figure 1 and Table 1): individuals with 12 chromosomes presented average genome sizes of 2.98 pg/2C (range: 2.84 – 3.10 pg/2C; Figures 1A,B), while individuals with 24 chromosomes had average genome sizes of 6.06 pg (range: 5.80–6.36 pg; Figures 1A,C), corresponding to diploid and tetraploid cytotypes, respectively.
FIGURE 1.
Cytogenetic diversity in Jasione maritima: (A) Flow cytometric histogram of relative propidium iodide fluorescence intensity (PI Fluorescence) of nuclei isolated from fresh leaves of S. lycopersicum “Stupické” (S.l.; reference standard with 2C = 1.96 pg) and of Jasione maritima diploid (2x) and tetraploid (4x) individuals; (B) Chromosome plate of a diploid J. maritima var. maritima individual from population MS003 (2n = 2x = 12 chromosomes; bar = 20 μm, Table 1); (C) Chromosome plate of a tetraploid J. maritima var. maritima individual from population SC116 (2n = 4x = 24 chromosomes; bar = 20 μm, Table 1). In A, for each peak, the mean relative fluorescence (Mean FL), DNA index (DI, Mean FL of J. maritima peak/Mean FL of the reference standard) and coefficient of variation of the peak (CV, in%) are provided.
TABLE 1.
Chromosome number and genome size variation in Jasione maritima.
| Taxon | Ploidy level | Chr. no. | Holoploid G.s. (2C, pg) | Monoploid G.s. (1Cx, pg) | Nind | Npop | |||||
| Mean | SD | CV (%) | Min | Max | Mean | SD | |||||
| J. maritima var. maritima | 2x | 12 | 2.98a | 0.07 | 2.4% | 2.84 | 3.10 | 0.25n.s. | 0.01 | 24 | 6 |
| 4x | 24 | 6.06b | 0.11 | 1.9% | 5.80 | 6.36 | 0.25n.s. | 0.01 | 38 | 6 | |
| J. maritima var. sabularia | 4x | 24 | 5.99b | 0.18 | 1.2% | 5.76 | 6.36 | 0.25n.s. | 0.01 | 22 | 3 |
DNA ploidy levels, chromosome number (2n, Chr. no.), holoploid (2C) and monoploid (1Cx) genome size are presented in picograms (pg). For genome size estimates, mean, standard deviation (SD), coefficient of variation (CV,%), minimum and maximum values are given. Different letter corresponds to statistically significant differences at P < 0.05; n.s. denotes no significant differences at P > 0.05. The columns Nind and Npop provide the number of analyzed individuals and populations, respectively.
Jasione maritima var. maritima presented both diploid and tetraploid individuals, while var. sabularia was tetraploid, only. Significant differences were observed in holoploid genome sizes (F2,81 = 5020, P < 0.001), with differences occurring between cytotypes (P < 0.05), but not between varieties within the tetraploid cytotype (P > 0.05; Table 1). Thus, tetraploids from the two varieties can only be distinguished based on morphologic traits. No statistically significant differences were observed between varieties and cytotypes regarding monoploid genome size (F2,81 = 0.534, P = 0.588; Table 1; Supplementary Table 6).
Cytotype Distributions in the Iberian Peninsula
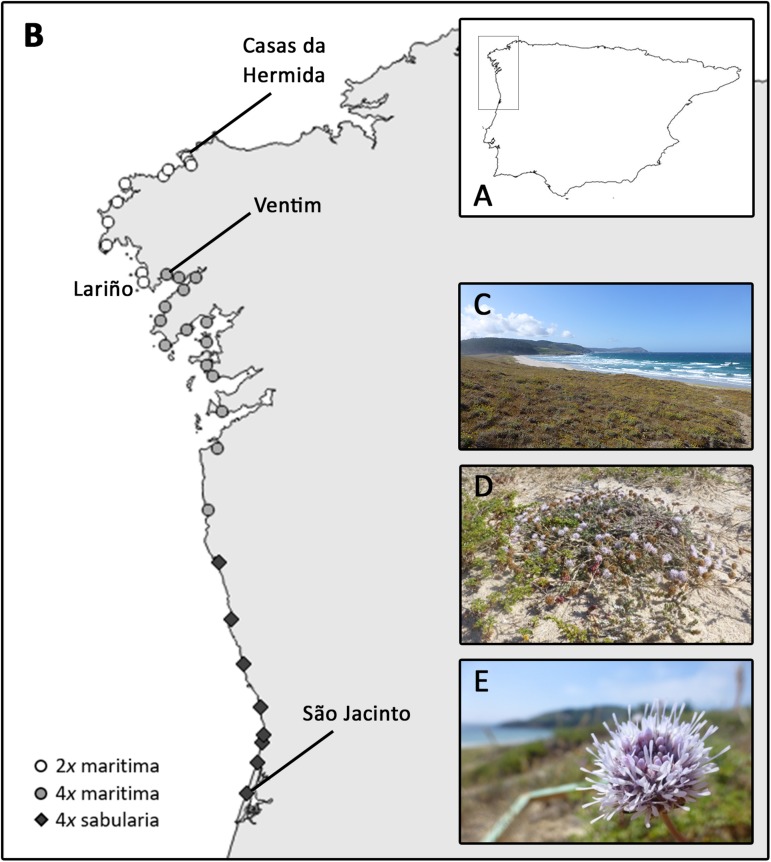
We assessed the DNA ploidy of 986 individuals from 35 natural populations spanning the distribution range of J. maritima in the Iberian Peninsula (Figure 2; Supplementary Table 1). The large-scale survey revealed diploid and tetraploid single-ploidy populations. As described above, J. maritima var. maritima comprises both diploids and tetraploids, while J. maritima var. sabularia is only tetraploid. The taxa and cytotypes are distributed parapatrically; diploid var. maritima occurs in the north, from Casas da Hermida to Lariño (Spain), tetraploid var. maritima occurs from Ventim (few kms south of the southern-most diploid population) to the south along the northwestern Spanish coast; and tetraploid var. sabularia occurs along the northern coast of Portugal (Figure 2). In the Iberian Peninsula, tetraploids occupy a wider area than diploids (Figure 2).
FIGURE 2.
Jasione maritima large-scale cytotype distribution based on flow cytometric analyses: (A) Iberian Peninsula with the screening area highlighted; (B) cytotype distribution in the surveyed area; (C) dune habitat, (D) habit, and (E) inflorescence of diploid J. maritima var. maritima of population SC150 (Table 1). Circles represent populations of J. maritima var. maritima, white correspond to diploids and light gray correspond to tetraploid populations, and dark gray diamonds refer to tetraploid J. maritima var. sabularia populations.
Environmental Niche
Univariate analyses of environmental variables for the entire distribution of J. maritima revealed significant differences for all variables except elevation (see Supplementary Table 2 for F and P values). Diploid var. maritima tends to occur in areas with lower temperatures and higher precipitation levels in the driest period (i.e., in summer) than tetraploid var. maritima and tetraploid var. sabularia, which both colonize warmer areas with drier summers (Supplementary Table 2). In particular, when compared with both tetraploid varieties, the diploid var. maritima occurs in areas with significantly lower annual mean temperature, mean temperatures of quarter periods, precipitation seasonality and annual precipitation, and although precipitation was significantly lower in wetter and colder periods, it was significantly higher in the driest and warmest periods (all P < 0.05; Supplementary Table 2). Tetraploid var. maritima occurs in climatic conditions that are, in general, more similar to the tetraploid var. sabularia than to diploids (Supplementary Table 2), namely for annual mean temperature, mean temperatures of quarter periods and precipitation of the wettest quarter (P > 0.05; Supplementary Table 2), not differing from diploid for isothermality and maximum temperature of the warmest month (P > 0.05; Supplementary Table 2). Interestingly, the areas occupied by the tetraploid var. maritima presented intermediate and significantly different values for precipitation variables (P < 0.05; except for precipitation in the wettest month and quarter, P > 0.05; Supplementary Table 2). The tetraploid var. sabularia occurs in areas with significantly higher precipitation seasonality, reflected in significantly higher precipitation in the coldest quarter, while having significantly lower precipitation in driest and warmest periods in comparison with both cytotypes of var. maritima (all P < 0.05; Supplementary Table 2). The PCA revealed two clusters of diploid populations; the French populations were clearly separated from the Iberian populations along axis 1 (high loadings for example for precipitation and temperature seasonality, Supplementary Figure 1A and Supplementary Table 4). In contrast, the diploid Iberian populations cluster near the tetraploid populations of both varieties (Supplementary Table 2 and Supplementary Figure 1A).
When analyzed at finer spatial resolution (100 m) in the Iberian Peninsula (the putative contact zone), significant differences among varieties and cytotypes were obtained for all variables except elevation, aspect and distance from the sea (see Supplementary Table 3 for F and P values). Diploid var. maritima colonizes areas with slightly higher elevation and facing west (although no significant differences were observed between cytotypes and varieties), and with significantly steeper slopes, lower topographic index, higher mean annual precipitation and lower mean summer temperatures (cooler areas) than tetraploid var. sabularia (all P < 0.05; Supplementary Table 3). The southern areas colonized by tetraploid var. sabularia are thus marked by significantly lower slopes (P < 0.05) facing south-southwest and by significantly hotter and drier environments than those occupied by diploids (i.e., significantly higher mean summer temperature and mean annual precipitation, P < 0.05; Supplementary Table 3). Tetraploid var. maritima tends to occur in areas environmentally similar to the diploids (i.e., similar mean summer temperatures and mean annual precipitation; P > 0.05), with the exception of slope values and topographic indexes, which were different from diploids (P < 0.05) and similar to those obtained in tetraploid var. sabularia localities (P > 0.05; Supplementary Table 3). Slope and topographic index suggest that the diploid var. maritima is usually located at interdunes depressions (low negative TPI and steeper slopes), while the tetraploids of both varieties usually grow in flat areas (TPI close to zero and low slopes). Consequently, in the PCA, the two cytotypes of var. maritima are clustered together and are separated from tetraploid var. sabularia along axes 1 and 2, which are associated with mean annual precipitation and mean summer temperature (Supplementary Tables 3, 4 and Supplementary Figure 1B).
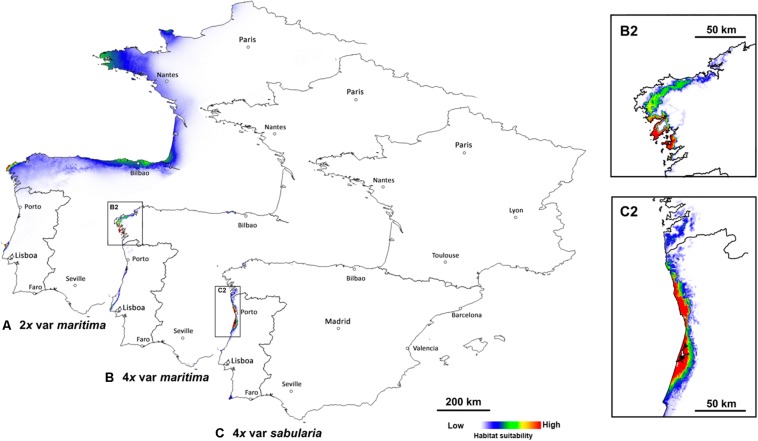
Predicted distributions based on niche modeling confirm the parapatric distributions of the three cytotypes (Figures 3, 4). Across the entire geographic distribution of J. maritima, models showed high habitat suitability for diploid var. maritima (Figure 3A) on the northwest coast of the Iberian Peninsula, the Death Coast of Spain where Iberian populations are currently located (Figure 2), in the south and northwest coast of France where the plant currently occurs, and on the coast near Bilbao, where no natural populations currently occur (Figure 3A). By contrast, the suitable habitats for tetraploids are restricted to the northwest of the Iberian Peninsula. Tetraploid var. maritima has a high probability of occurrence on the coast between Lariño and A Guarda in Spain (Figure 3B), corresponding to its current distribution (Figure 2). Tetraploid var. sabularia has a high likelihood of occurrence on the northern Portuguese coast, from Viana do Castelo to Figueira da Foz (Figure 3C), matching its current distribution, but also further south in areas where its presence was not detected.
FIGURE 3.
Predicted geographic niches for (A) diploids of Jasione maritima var. maritima (2x var. maritima), (B) tetraploids of J. maritima var. maritima (4x var. maritima), plus an inset of the area with higher probability of occurrence (B2), and (C) tetraploids of J. maritima var. sabularia (4x var. sabularia), plus an inset of the area with higher probability of occurrence (C2), considering the entire distribution area. White to red color gradient represent habitats with higher and lower probability of occurrence of the cytotype, respectively.
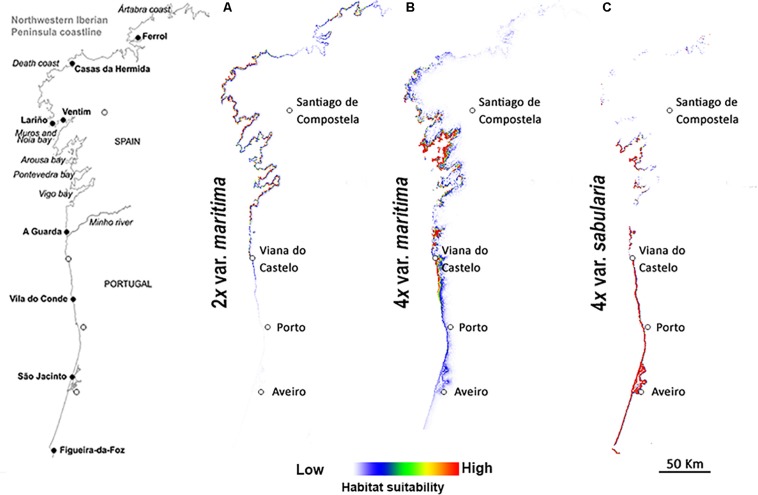
FIGURE 4.
Predicted geographic niches for (A) diploids of Jasione maritima var. maritima, (B) tetraploids of Jasione maritima var. maritima, and (C) tetraploids of Jasione maritima var. sabularia, in the sympatric zones at the Iberian Peninsula. White to red color gradient represent habitats with higher and lower probability of occurrence of the cytotype, respectively.
The results for the putative contact zone revealed similar patterns (Figure 4). Suitable habitat for diploid var. maritima includes its current distribution range but extends further south along the Spanish coast (Figure 4A), overlapping the current distribution of tetraploid var. maritima. Suitable habitat for diploids also occurs around Costa Ártabra, but we did not detect natural populations in these coastal regions. The highest suitability for tetraploid var. maritima is found around the bay of Arousa and scattered also in the bays of Muros and Noia, corresponding to its current northern distribution (where it contacts with the diploid var. maritima; Figures 2, 4). Suitable habitats for tetraploid var. maritima also occur further south, particularly in southernmost regions of the Spanish coast (where it contacts with the tetraploid var. sabularia; Figures 2, 4) and in northernmost parts of the Portuguese coast (Figure 4B), where its presence was not detected (Figure 2). In the case of the tetraploid var. sabularia, suitable habitat extends beyond the observed distribution, to the north, where tetraploid var. maritima is currently found, and especially to the south (Figure 4C), where no J. maritima has been found (Figures 2, 4C).
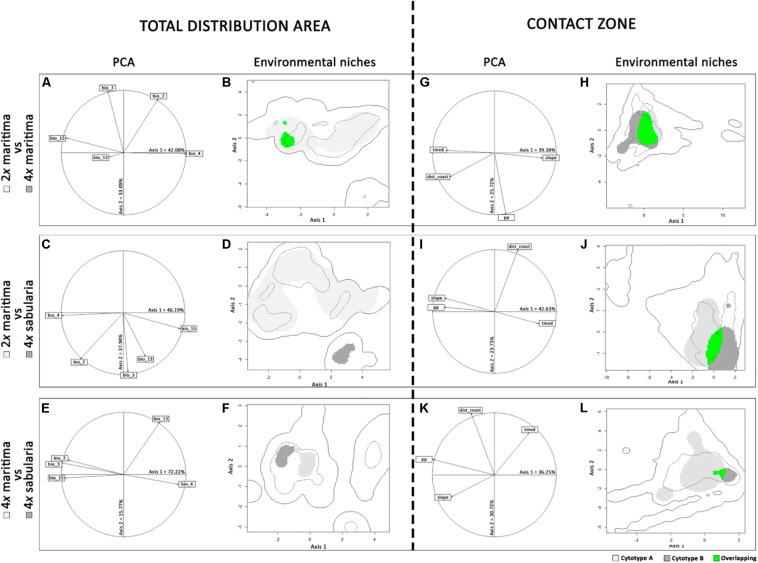
The first two axes of the PCAs explained a high percentage of environmental variance, namely over 75% for the entire distribution, and over 65% for the Iberian Peninsula (Figure 5; Supplementary Table 7). Across the entire species distribution range (Figures 5A–F), the environmental niche of tetraploid var. maritima falls completely within the environmental range of the diploid var. maritima (Figure 5B). Conversely, the amplitude of the environmental niche of diploids was much larger than that of the tetraploids, and only 6.9% of the diploid var. maritima environmental niche overlaps with the tetraploid var. maritima niche (dark gray area in Figure 5B). The environmental niche of tetraploid var. sabularia is distinguishable from diploid var. maritima mainly along axis 2, associated with isothermality and precipitation of the wettest month (Figures 5C,D and Supplementary Table 8), and from tetraploid var. maritima along axis 1, with strong associations with most of the selected variables (Figures 5E,F).
FIGURE 5.
Ecological niche models for the varieties and cytotypes of Jasione maritima at: (A–F) the entire distribution area and (G–L) the Iberian Peninsula. (A), (C), (E), (G), (I), and (K) represent the contribution of selected environmental variables [mean diurnal range (bio_2), isothermality (bio_3), temperature seasonality (bio_4), precipitation of the wettest month (bio_13) and precipitation seasonality (bio_15), slope, mean summer temperature (tmed), mean annual precipitation (pp) and distance to coast (dist)] to the first two axes of the principal component analyses (PCA), with the percentage of variance explained by each axis provided in each graphic; (B), (D), (F), (H), (J), and (K) represent the environmental niche of diploid J. maritima var. maritima (2x maritima), tetraploid J. maritima var. maritima (4x maritima) and tetraploid J. maritima var. sabularia (4x sabularia), compared in pairs; colored areas represent the following: light gray – suitable habitats for variety/cytotype A, dark gray – suitable habitats for variety/cytotype B, and green – denotes the overlapping areas between cytotype A and B environmental niches; variety/cytotype A and B can be identified in the left of each panel; the continuous line corresponds to the whole climatic space, while the dashed line indicates the 75th percentile.
Analyses of the putative contact zone in the Iberian Peninsula (Figures 5G–L) show that the environmental ranges of the diploid and tetraploid cytotypes of var. maritima partially overlap; in particular, 68.0% of the diploid environmental niche overlaps with the tetraploid niche, and 53.7% for the reverse comparison (Figure 5H). The overlap between tetraploid var. sabularia and either cytotype of var. maritima is considerably lower (Figures 5J,L; Supplementary Table 7): 21.0% of the diploid var. maritima environmental niche and 3.6% of the tetraploid var. maritima niche overlaps with that of tetraploid var. sabularia; whereas 33.4 and 34.5% of the tetraploid var. sabularia environmental niche overlaps with diploid and tetraploid var. maritima, respectively. Also, the amplitude of the environmental niche of tetraploid var. sabularia was smaller, especially in comparison with that of tetraploid var. maritima (Figure 5L).
Discussion
The niche shift hypothesis proposes that ecological divergence of polyploid lineages is a key process shaping the distribution of cytotypes in nature (Levin, 1975; Fowler and Levin, 1984). Here we provide detailed cytogeographical information for the two J. maritima varieties, diploid-tetraploid var. maritima and tetraploid var. sabularia, and explore the relationships between their environmental requirements and current distributions. Whereas tetraploid var. sabularia differed environmentally from diploid var. maritima, suggesting niche divergence, tetraploid var. maritima overlapped with the diploid, suggesting that other factors rather than niche divergence were involved in current distribution of tetraploid var. maritima. Additionally, tetraploid var. maritima currently occupies its entire predicted range, whereas diploids are restricted to the northern portion of their suitable environment only. Below we discuss these findings and its implications for the dynamics of diploid-polyploid complexes.
Parapatric Distribution of J. maritima Varieties and Cytotypes
The current distributions of J. maritima varieties and cytotypes are clearly geographically segregated. Our chromosome counts revealed for the first time the presence of diploid individuals (2n = 2x = 12) in the northernmost localities of the Iberian Peninsula, which are confirmed by their lower genome size estimates (2.98 ± 0.07 pg) compared to tetraploids (6.06 ± 0.11 pg). Prior to this work, J. maritima on the Iberian Peninsula was considered to be only tetraploid (2n = 4x = 24; Lago Canzobre and Castroviejo, 1992; Rubido-Bará et al., 2010). Therefore, diploid var. maritima has a disjunct geographic distribution comprising the west coast of France and the northwest Iberian Peninsula. Although close affinities between the French and the Iberian populations have been pointed out (Parnell, 1987), the populations from these two regions differ significantly in their environmental variables. Such differences may reflect ecological divergence in allopatry and/or different evolutionary pathways. Further molecular studies are needed to unravel the relationships between French and Iberian populations.
Within the Iberian Peninsula, our survey revealed that the cytotypes and varieties are distributed parapatrically, with diploids observed in the north and tetraploids occupying more southern locations over an area that is larger than the one occupied by the diploids in this region. Two parapatric contact zones were observed, one between diploids and tetraploids of var. maritima in Lariño, within which populations of each cytotype are separated by a minimum of 8 kms, and another between tetraploids of var. maritima and sabularia at the Spanish-Portuguese border (Minho river separating var. maritima at north and var. sabularia at south). Spatial segregation between cytotypes has been observed in several other polyploid complexes (e.g., Husband and Schemske, 1998; Balao et al., 2009; Kolář et al., 2009; Sonnleitner et al., 2010; Castro et al., 2012, 2018; Casazza et al., 2016; Wefferling et al., 2017). Spatial segregation reduces inter-cytotype interactions and constitutes a physical barrier that prevents gene flow between cytotypes (Segraves and Thompson, 1999; Husband and Schemske, 2000; Baack, 2005; Nuismer and Cunningham, 2005) and weakens frequency-dependent selection on minority cytotypes (Levin, 1975). Consequently, it is widely viewed as a significant contributor to escape direct competition with diploid counterparts (Levin, 2002; Li et al., 2004; Baack and Stanton, 2005). Parapatric distribution may reflect competitive interactions or frequency dependent mating disadvantage, which prevent one cytotype from invading another cytotype area (see below). Reproductive isolation among J. maritima cytotypes and varieties mediated by current spatial segregation might promote evolutionary divergence, especially if the cytotypes and varieties are subjected to different selective pressures across the latitudinal range occupied by the species. In the long term, this could lead to the formation of distinct taxa (Otto and Whitton, 2000; Soltis et al., 2010).
Environmental Niche Shifts
The north-south sequential linear distribution of J. maritima cytotypes and varieties fits the coastline of a transitional biogeographic unit between the Mediterranean and Atlantic regions (the Galicia and North Portugal biogeographic sector; Rivas-Martínez et al., 2017) and a limit to the distribution of several species. The non-overlapping ranges support a process of differentiation along an environmental gradient with each variety and cytotype occupying a subset of environmental conditions along a thermal and precipitation gradient representing an increase of Mediterranean conditions from north to south. In this context, the tetraploids of the two J. maritima varieties revealed different degrees of environmental divergence in comparison with their diploid counterpart. Our analyses indicate that the environmental niche of tetraploid var. sabularia is distinguishable from both diploid and tetraploid var. maritima cytotypes. In particular, the tetraploid var. sabularia colonized areas facing S-SW, which are hotter and slightly drier environments than those colonized by the var. maritima. These environmental differences result in little environmental niche overlap between the tetraploid var. sabularia and both cytotypes of var. maritima, suggesting that tetraploid var. sabularia has different tolerances to water availability and temperature, which allowed them to colonize areas beyond those suitable for the diploids. The capacity of polyploids to grow in environments that differ from their progenitor(s) allows polyploid lineages to expand to new areas where their lower ploidy parental(s) are absent (Maherali et al., 2009; Laere et al., 2011; Manzaneda et al., 2012, 2015; Hao et al., 2013) and, thus, avoid minority cytotype exclusion (Levin, 1975; Fowler and Levin, 1984; Felber, 1991). While we do not know whether the ecological differences arose in association with genome duplication or after separation from diploid var. maritima, our results are consistent with the importance of niche differentiation for successful establishment of polyploids. Further studies assessing physiological responses under contrasting water availabilities and temperatures are needed to experimentally assess their impacts on cytotype fitness.
In contrast to var. sabularia, the environmental niche of tetraploid var. maritima overlaps with the environmental niche of diploids. Additionally, the geographical distributions and the high-resolution analyses of habitat suitability at the putative contact zone shows that diploids seem to be confined to the northern suitable places, while the tetraploids occupy most of their suitable areas. Thus, although some degree of niche expansion is detected, there is poor evidence that whole genome duplications that led to the formation of the tetraploid var. maritima and/or posterior processes of divergence resulted in a shift in environmental niche in comparison with the diploid, and other factors rather than niche divergence must have been involved in the successful spread of tetraploid var. maritima. Interestingly, tetraploids of var. maritima invest more in belowground biomass than diploids, a trait that could have provided an advantage to colonize southern locations more severely affected by drought in comparison with northern locations where diploids occur (Castro, 2018). In the closely related diploid-tetraploid J. montana, environmental niche analyses also revealed that the tetraploid niche was nested within the diploid niche breadth, and environmental sorting was proposed as a determinant for their successful establishment (Castro et al., 2019). Although the occurrence of niche shifts in association with polyploidization has been described in some diploid-polyploid systems (e.g., Hao et al., 2013; Thompson et al., 2014; Visger et al., 2016; Muñoz-Pajares et al., 2018), there is also evidence in the literature supporting the idea that polyploids and their diploid progenitors occupy similar environmental conditions (e.g., Godsoe et al., 2013; Castro et al., 2019; reviewed in Glennon et al., 2014; Spoelhof et al., 2017). This supports the hypothesis that the success of the tetraploids might be context-dependent and species – or even genotype – specific. Thus, niche divergence could have been key in certain systems while in other processes, alone or combined, could have contributed for the current distribution patterns of the tetraploid var. maritima.
Current Distribution Patterns at the Zones of Sympatry
While in northernmost areas environmental variables seem to explain the current distribution range of tetraploid var. maritima, other factors may prevent diploids from thriving within the tetraploid’s environmental niche. Increased competitive ability of polyploids may have allowed them to overcome frequency-dependent selection (Levin, 1975; Fowler and Levin, 1984; Rodríguez, 1996) and exclude diploids from invading these sites. Evidence for strong polyploid competitive ability in other species is conflicting (e.g., Maceira et al., 1993; Collins et al., 2011; Thompson et al., 2015). For example, tetraploid Dactylis glomerata is highly competitive compared to diploids (Maceira et al., 1993), whereas diploid and tetraploid C. angustifolium (Thompson et al., 2015) have similar competitive abilities. Relative competitive abilities of diploid and tetraploid Centaurea stoebe vary across the distribution range (Collins et al., 2011). In J. maritima, similar competitive abilities may promote a stable contact zone, whereas different competitive abilities are expected to generate a moving contact zone (e.g., Maceira et al., 1993), toward the south if diploids are more competitive, or to the north if tetraploids are stronger competitors, in any case expanding cytotype area until the environmental limit of the strongest competitor is reached. Similarly, in the southernmost zone of sympatry between tetraploid varieties, competitive ability may also have played an important role in the dynamics of the putative contact zone, as models predict that tetraploid var. maritima could occur further south in Portuguese coast and tetraploid var. sabularia could occur further north in Spanish territory. Therefore, competition experiments are needed to evaluate the role of competitive ability in shaping current cytotype distribution patterns in J. maritima.
Alternatively, reduced dispersal ability could limit the expansion of diploids to locations where tetraploid var. maritima grows. The seeds of Jasione species have no obvious dispersal mechanism. Like the seeds of J. montana, those of J. maritima are completely smooth; they are not adapted to water dispersal, as they immediately sink (Praeger, 1913; M. Castro, personal observations), nor to endozoochory, as it detrimentally affects germination levels (Peco et al., 2006). Previous experiments have also shown that the maximum seed dispersal distance seldom exceeds 1.4 m in J. montana (Parnell, 1985). Seed dispersal in J. maritima seems also to be hindered by the coast line characteristics, namely the restricted linear shape of dune habitat; the existence of spatial discontinuity in habitat associated with the occurrence of rocky outcrops reaching the coast and interrupting the dune system; the existence of deep gulfs, especially in the NW coast of Spain; and the presence of large end sections of rivers with significant discharge along the year (e.g., Minho river, or the wide Aveiro coastal lagoon-estuarine system at the current southern limit of var. sabularia). Nevertheless, even if diploids were capable of dispersing to southern locations, they may not find a suitable site to establish or may experience strong frequency dependent selection within tetraploid populations (Levin, 1975; Husband, 2000), particularly being a self-incompatible plant (Siopa et al., 2020). Occasional migration from the diploid area into the tetraploid range may allow some rates of gene flow (e.g., through triploid bridge) that could attenuate divergence in environmental requirements between cytotypes of var. maritima. In the absence of other fitness advantages, diploids would be eliminated from tetraploid populations and the current parapatric distribution maintained.
Hypothesis About the Origins of the Two Tetraploids and Implications for Biodiversity
The evolutionary history of Jasione is still largely unknown. The few available phylogenetic studies suggest a recent origin of the species within the genus, linked with Pleistocene glaciation (Sales et al., 2004; Pérez-Espona et al., 2005). Consequently, we have limited evidence on which to build hypotheses regarding the origin of the tetraploids. J. maritima is closely related to the widespread J. montana (Bokhari and Sales, 2001; Pérez-Espona et al., 2005), and French and Spanish diploid populations have been considered a coastal sand-dune form of the inland J. montana (Parnell, 1980, 1982), although other authors have stressed the differences between J. maritima and some coastal forms of J. montana (Dupont, 2015). The data available for the Iberian Peninsula points that, on one hand, the diploids and tetraploids of var. maritima are very difficult to differentiate morphologically (Sales and Hedge, 2001), and were shown here to share similar environmental niches, two features usually associated with autopolyploid complexes (e.g., Glennon et al., 2012, 2014; Godsoe et al., 2013). On the other hand, the tetraploid var. sabularia, although closely resembling var. maritima in morphological and anatomical characters (Bokhari and Sales, 2001), exhibits some subtle morphological differences, such as sparser indumentum and different leaf shape that have led some taxonomists to treat it separately (e.g., as J. lusitanica in Flora Europaea; Tutin et al., 1976). In addition, genetic analyses using AFLPs separated var. sabularia from var. maritima and the closely related J. montana (Pérez-Espona et al., 2005). Unfortunately, based on the sampling locations, those studies likely only included diploid var. maritima. This previous research, together with the detection of niche shifts in comparison with the diploids, could suggest a separate divergence from the diploid parental or an allopolyploid origin of J. maritima var. sabularia. Allopolyploids often show either intermediate morphological features between putative parental diploids or transgressive phenotypes (Soltis et al., 2007), and this could the case of var. sabularia. Still, it is important to note that the different possibilities for the origins of the tetraploid varieties complicate the interpretation of niche divergence relative to the diploid var. maritima that span such great ecological space.
The origins of J. maritima seem compatible with a diversification model by which glacial refugia in the Iberian coast would have acted as triggers of speciation (e.g., González-Sampériz et al., 2010; Fernández-Mazuecos and Vargas, 2013). During this period, an ecotype of J. montana adapted to maritime sand-dunes could have survived in these coastal areas, and with the temperature fluctuation given rise to tetraploid cytotypes (once or multiple times); the tetraploids could have arisen through autopolyploidization or after hybridization with other taxa (allopolyploidization). At the current state of knowledge, we can only propose hypotheses, and phylogenetic and phylogeographic studies are necessary to test them and shed light on the timing and frequency of whole genome duplications in the genus. That said, our analyses of cytotype distribution and environmental niche variation within J. maritima polyploidy complex set a framework for appropriate hypothesis testing, and the detected ecological differences can contribute to understanding the internal biodiversity in the complex. In J. maritima, populations differing in ploidy can exhibit niche differences, as in tetraploid var. sabularia vs. diploid var. maritima, or differences in niche occupancy and geographical space, for which differences in competitive abilities could be invoked (Laport et al., 2013), as in tetraploid var. maritima vs. diploid var. maritima. Ultimately, each cytotype and variety probably has unique responses to the abiotic environment and to biotic interactions (Segraves and Anneberg, 2016), having clearly separate ranges that support the idea that they work as different functional units of biodiversity (Ramsey and Ramsey, 2014; Laport and Ng, 2017). Despite traditional reluctance to incorporate intraspecific ploidy differences into taxonomic decisions, particularly when no striking morphological differences are exhibited, as is frequent in autopolyploids (Soltis et al., 2007), our results support the recognition of diverse biodiversity units both between cytotypes and within the tetraploid cytotype in the J. maritima complex. Therefore, the study of differences and similarities in ecological envelopes among ploidy units is a valuable tool and should inform taxonomic and management decisions, particularly when the response to environmental factors (e.g., climate change) could be specific of each of the different units.
Conclusion
In this study, we show that J. maritima has two parapatric contact zones in the Iberian Peninsula along a linear coastal distribution, one between diploid and tetraploid populations of the same variety (var. maritima), and the other between tetraploids from two different varieties (var. maritima and var. sabularia). The two tetraploids have different degrees of environmental divergence in comparison with the diploid. Whereas changes in environmental requirements may have allowed tetraploid var. sabularia to spread in habitats not favorable to its diploid progenitor, other factors such as ploidy interactions and competitive ability, have influenced the spread of tetraploid var. maritima. More studies, such as phylogenetic and phylogeographic reconstructions, and reciprocal transplants, competition and drought experiments, are needed to test these hypotheses. Differences in ecological tolerances can suggest the presence of different functional units of biodiversity within polyploid groups, allowing the study of factors related to post-polyploidization divergence.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
MC, JL, MS, and SC designed the experiment and conducted field collections. MC conducted laboratory analysis. MC, AF, and SC performed the niche modeling analyses. All authors participated in scientific discussion and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Ana Caperta, Leonor Morais and Daniela Tavares for all the helpful methodological suggestions and assistance in chromosome counts. Permits to collect samples and undertake scientific research were obtained from ICNF – Instituto da Conservac̨ão da Natureza e das Florestas (Portugal), reference 866, and Xunta de Galicia – Consellería de Medio Ambiente e Ordenación do Territ rio (Spain), reference 12150/RX1618202. The authors are also extremely thankful to all Reviewers for all the constructive and helpful comments, all of which enabled to significantly improve the work.
Funding. This research was supported by POPH/FSE funds by the Portuguese Foundation for Science and Technology (FCT) with a doctoral grant to MC (SFRH/BD/89910/2012), a starting grant and exploratory project to SC (IF/01267/2013) and the project UID/BIA/04004/2020, by Xunta de Galicia with the grant to MS (ED431B 2018/36), and by Project RENATURE financed by the “Programa Operacional Regional do Centro 2014-2020 (Centro2020) – CENTRO-01-0145-FEDER-000007”.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00315/full#supplementary-material
Principal component analyses: (A) for the entire geographic distribution of J. maritima (in Portugal, Spain and France), using 19 bioclimatic variables (Bio1-Bio19) plus elevation extracted for all species occurrences from the Worldclim database at a resolution of 1 km; (B) for the contact zone (in the northwest Iberian Peninsula), using the following set of variables at 100 m resolution – elevation (ele); aspect, slope, slope range (slprng) and topographic position index (tpi); summer mean temperature (tmed) and mean annual precipitation (pp); lithology (lito); and distance to coast (dist_coast). Variance explained by each axis is also provided.
Geographic information of the Jasione maritima populations sampled in this study for flow cytometric analyses. For each population, an ID code, locality name, herbarium code at SANT, geographical coordinates (angular), sample size (N, number of individuals analyzed) and estimated DNA ploidy level are given. Ploidy levels: diploids (2x) and tetraploids (4x). The two ID codes marked with an asterisk (∗) denote populations where seeds were collected for chromosome counts.
Environmental variables characterization in Jasione maritima considering the total distribution area. For each environmental variable mean and standard error of the mean (se) per cytotype are presented. F and P values are also presented. Different letter corresponds to statistically differences (P < 0.05) between the groups for a given environmental variable. Shades highlight differences between cytotypes/varieties. Bold highlight variables used in niche modeling.
Environmental variables characterization in Jasione maritima in the contact zone (northwest of Iberian Peninsula) area. For each variable mean and standard error of the mean (se) are presented. F and P values are also presented. Different letter corresponds to statistically differences (P < 0.05) between the group within the same environmental variable. Shades highlight differences between cytotypes/varieties. Bold highlight variables used in niche modeling.
Variable contribution in Principal Component Analyses using all variables (Figure 1) at total distribution area and contact zone analyses.
Jasione maritima models’ evaluation. For diploid individuals of J. maritima var. maritima (2x var. maritima), tetraploid individuals of J. maritima var. maritima (4x var. maritima) and tetraploid individuals of J. maritima var. sabularia (4x var. sabularia) mean and standard error of AUC values of the models and of omission rates were presented for each cytotype in the different approaches. The value of omission rate of the final model for each cytotype/approach were also presented.
Genome size estimates in Jasione maritima. In each population, DNA ploidy estimates and mean, standard deviation of the mean (SD), coefficient of variation (CV, in %), minimum (Min) and maximum (Max) values of holoploid genome size (2C, in pg) are given. Number of individuals analyzed for genome size in each population (n) and mean monoploid genome size (1Cx, in pg) are also provided. Ploidy levels: diploids (2x) and tetraploids (4x).
Jasione maritima environmental niches overlapping comparing the two varieties and two cytotypes in pairs (diploid niche of J. maritima var. maritima – 2x var. maritima, tetraploid niche of J. maritima var. maritima – 4x var. maritima and tetraploid niche of J. maritima var. sabularia – 4x var. sabularia). The percentage of variance explained the two-principal axis, the total of variance explained, percentage of environmental niche overlapping of cytotype A niche in cytotype B niche (A → B) and cytotype B niche in cytotype A niche (B → A) were present for each comparison in the two approaches.
Variable contribution in Principal Component Analyses using selected variables using in the models (Figure 5) at total distribution area and contact zone analyses.
References
- Araújo M. B., New M. (2007). Ensemble forecasting of species distributions. Trends Ecol. Evol. 22 42–47. 10.1016/j.tree.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Aseginolaza C., Gómez D., Lizaur X., Montserrat-martí G., Morante G., Salaverría M. R., et al. (1984). Catálogo Florístico de Álava, Vizcaya y Guipúzcoa. Vitoria: Gobierno Vasco. [Google Scholar]
- Baack E. J. (2004). Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae). Am. J. Bot. 91 1783–1788. 10.3732/ajb.91.11.1783 [DOI] [PubMed] [Google Scholar]
- Baack E. J. (2005). To succeed globally, disperse locally: effects of local pollen and seed dispersal on tetraploid establishment. Heredity 94 538–546. 10.1038/sj.hdy.6800656 [DOI] [PubMed] [Google Scholar]
- Baack E. J., Stanton M. L. (2005). Ecological factors influencing tetraploid speciation in snow buttercups (Ranunculus adoneus): niche differentiation and tetraploid establishment. Evolution 59 1936–1944. 10.1111/j.0014-3820.2005.tb01063.x [DOI] [PubMed] [Google Scholar]
- Balao F., Casimiro-Soriguer R., Talavera M., Herrera J., Talavera S. (2009). Distribution and diversity of cytotypes in Dianthus broteri as evidenced by genome size variations. Ann. Bot. 104 965–973. 10.1093/aob/mcp182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2014). lme4: Linear Mixed-Effects Models Using Eigen and S4. Available online at: http://CRAN.R-project.org/package=lme4 (accessed February 2018). [Google Scholar]
- Bennett M. D. (1998). Plant genome values: how much do we know? Proc. Natl. Acad. Sci. U.S.A. 95 2011–2016. 10.1073/pnas.95.5.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari M. H., Sales F. (2001). Jasione (Campanulaceae) anatomy in the Iberian Peninsula and its taxonomic significance. Edinb. J. Bot. 58 405–422. 10.1017/S0960428601000725 [DOI] [Google Scholar]
- Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24 127–135. 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Brochmann C., Brysting A. K., Alsos I. G., Borgen L., Grundt H. H., Scheen A. C., et al. (2004). Polyploidy in arctic plants. Biol. J. Lin. Soc. 82 521–536. 10.1111/j.1095-8312.2004.00337.x [DOI] [Google Scholar]
- Broennimann O., Fitzpatrick M. C., Pearman P. B., Petitpierre B., Pellissier L., Yoccoz N. G., et al. (2012). Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21 481–497. 10.1111/j.1466-8238.2011.00698.x [DOI] [Google Scholar]
- Buggs R. J., Pannell J. R. (2007). Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 61 125–140. 10.1111/j.1558-5646.2007.00010.x [DOI] [PubMed] [Google Scholar]
- Casazza G., Boucher F. C., Minuto L., Randin C. F., Conti E. (2016). Do floral and niche shifts favour the establishment and persistence of newly arisen polyploids? A case study in an Alpine primrose. Ann. Bot. 119 81–93. 10.1093/aob/mcw221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M. (2018). Evolutionary Ecology of Polyploids: Understanding Species Coexistence at the Contact Zones. Dissertation, University of Coimbra, Coimbra. [Google Scholar]
- Castro M., Castro S., Figueiredo A., Husband B. C., Loureiro J. (2018). Complex cytogeographical patterns reveal a dynamic tetraploid-octoploid contact zone. AoB Plants 10 1–18. 10.1093/aobpla/ply012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M., Loureiro J., Serrano M., Tavares D., Husband B. C., Siopa C., et al. (2019). Mosaic distribution of cytotypes in a mixed-ploidy plant species, Jasione montana: nested environmental niches but low geographical overlap. Bot. J. Linn. Soc. 190 51–66. 10.1093/botlinnean/boz007 30541055 [DOI] [Google Scholar]
- Castro S., Loureiro J., Procházka T., Münzbergová Z. (2012). Cytotype distribution at a diploid–hexaploid contact zone in Aster amellus (Asteraceae). Ann. Bot. 110 1047–1055. 10.1093/aob/mcs177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. R., Naderi R., Mueller-Schaerer H. (2011). Competition between cytotypes changes across a longitudinal gradient in Centaurea stoebe (Asteraceae). Am. J. Bot. 98 1935–1942. 10.3732/ajb.1100063 [DOI] [PubMed] [Google Scholar]
- Delay J. (1967). Halophytes 1. Informations annuelles de caryostematique et cytogenetique. Travaux laboratoires de Phytogenetique, Strasbourg et Lille 1 11–12. [Google Scholar]
- Di Cola V., Broennimann O., Petitpierre B., Breiner F. T., D’amen M., Randin C., et al. (2017). ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography 40 774–787. 10.1111/ecog.02671 [DOI] [Google Scholar]
- Doležel J., Greilhuber J., Suda J. (2007). Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2 2233–2244. 10.1038/nprot.2007.310 [DOI] [PubMed] [Google Scholar]
- Doležel J., Sgorbati S., Lucretti S. (1992). Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 85 625–631. 10.1034/j.1399-3054.1992.850410.x [DOI] [Google Scholar]
- Dupont P. (2015). Les Plantes Vasculaires Atlantiques, les Pyrénéo-Cantabriques et les Éléments Floristiques Voisins Dans la Péninsule Ibérique et en France. Jarnac: Société botanique du centre-ouest. [Google Scholar]
- Felber F. (1991). Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. J. Evol. Biol. 4 195–207. 10.1046/j.1420-9101.1991.4020195.x [DOI] [Google Scholar]
- Fernández-Mazuecos M., Vargas P. (2013). Congruence between distribution modelling and phylogeographical analyses reveals Quaternary survival of a toadflax species (Linaria elegans) in oceanic climate areas of a mountain ring range. New Phytol. 198 1274–1289. 10.1111/nph.12220 [DOI] [PubMed] [Google Scholar]
- Fowler N. L., Levin D. A. (1984). Ecological constraints on the establishment of a novel polyploid in competition with its diploid progenitor. Am. Nat. 124 703–711. 10.1086/284307 [DOI] [Google Scholar]
- Fox J., Weisberg S., Adler D., Bates D., Baud-Bovy G., Ellison S. (2015). car: Companion to Applied Regression. Available online at: http://CRAN.R-project.org/package=car (accessed February 2018). [Google Scholar]
- Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. (1983). Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220 1049–1051. 10.1126/science.220.4601.1049 [DOI] [PubMed] [Google Scholar]
- Glennon K. L., Rissler L. J., Church S. A. (2012). Ecogeographic isolation: a reproductive barrier between species and between cytotypes in Houstonia (Rubiaceae). Evol. Ecol. 26 909–926. 10.1007/s10682-011-9539-x [DOI] [Google Scholar]
- Glennon K. L., Ritchie M. E., Segraves K. A. (2014). Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecol. Lett. 17 574–582. 10.1111/ele.12259 [DOI] [PubMed] [Google Scholar]
- Godsoe W., Larson M. A., Glennon K. L., Segraves K. A. (2013). Polyploidization in Heuchera cylindrica (Saxifragaceae) did not result in a shift in climatic requirements. Am. J. Bot. 100 496–508. 10.3732/ajb.1200275 [DOI] [PubMed] [Google Scholar]
- Goldblatt P., Takei M. (1993). Chromosome cytology of the African genus Lapeirousia (Iridaceae-Ixioideae). Ann. Mo. Bot. Gard. 80 961–973. 10.2307/2399939 [DOI] [Google Scholar]
- González-Sampériz P., Leroy S. A., Carrión J. S., Fernández S., García-Antón M., Gil-García M. J., et al. (2010). Steppes, savannahs, forests and phytodiversity reservoirs during the Pleistocene in the Iberian Peninsula. Rev. Palaeobot. Palynol. 162 427–457. 10.1016/j.revpalbo.2010.03.009 [DOI] [Google Scholar]
- Greilhuber J., Doležel J., Lysak M. A., Bennett M. D. (2005). The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Ann. Bot. 95 255–260. 10.1093/aob/mci019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J., Ebert I. (1994). Genome size variation in Pisum sativum. Genome 37 646–655. 10.1139/g94-092 [DOI] [PubMed] [Google Scholar]
- Greilhuber J., Temsch E. M., Loureiro J. (2007). “Nuclear DNA content measurement,” in Flow Cytometry With Plant Cells: Analysis of Genes, Chromosomes and Genomes, eds Dolezel J., Greilhuber J., Suda J. (Weinheim: Wiley-VCH; ), 67–101. 10.1002/9783527610921.ch4 [DOI] [Google Scholar]
- Guinea E. (1949). Vizcaya y su Paisaje Vegetal (Geobotánica vizcaina). Bilbao: Junta de Cultura de Vizcaya. [Google Scholar]
- Haberle R. C., Dang A., Lee T., Peñaflor C., Cortes-Burns H., Oestreich A., et al. (2009). Taxonomic and biogeographic implications of a phylogenetic analysis of the Campanulaceae based on three chloroplast genes. Taxon 58 715–734. 10.1002/tax.583003 [DOI] [Google Scholar]
- Hao G. Y., Lucero M. E., Sanderson S. C., Zacharias E. H., Holbrook N. M. (2013). Polyploidy enhances the occupation of heterogeneous environments through hydraulic related trade-offs in Atriplex canescens (Chenopodiaceae). New Phytol. 197 970–978. 10.1111/nph.12051 [DOI] [PubMed] [Google Scholar]
- Hijmans R. J., van Etten J., Cheng J., Mattiuzzi M., Sumner M., Greenberg J. A., et al. (2017). Package ‘raster’. Available at: https://cran.r-project.org/package=raster [Google Scholar]
- Hothorn T., Bretz F., Westfall P., Heiberger R. M. (2017). Multcomp: Simultaneous Inference for General Linear Hypotheses. Available online at: http://CRAN.Rproject.org/package=multcomp (accessed February 2018). [Google Scholar]
- Husband B. C. (2000). Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proc. R. Soc. Lond. B Biol. Sci. 267 217–223. 10.1098/rspb.2000.0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband B. C., Baldwin S. J., Suda J. (2013). “The incidence of polyploidy in natural plant populations: major patterns and evolutionary processes,” in Flow Cytometry With Plant Cells: Analysis of Genes, Chromosomes and Genomes, eds Dolezel J., Greilhuber J., Suda J. (Weinheim: Wiley-VCH; ), 255–276. 10.1007/978-3-7091-1160-4_16 [DOI] [Google Scholar]
- Husband B. C., Schemske D. W. (1998). Cytotype distribution at a diploid–tetraploid contact zone in Chamerion (Epilobium) angustifolium (Onagraceae). Am. J. Bot. 85 1688–1694. 10.2307/2446502 [DOI] [PubMed] [Google Scholar]
- Husband B. C., Schemske D. W. (2000). Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. J. Ecol. 88 689–701. 10.1046/j.1365-2745.2000.00481.x [DOI] [Google Scholar]
- Jiao Y., Wickett N. J., Ayyampalayam S., Chanderbali A. S., Landherr L., Ralph P. E., et al. (2011). Ancestral polyploidy in seed plants and angiosperms. Nature 473 97–100. 10.1038/nature09916 [DOI] [PubMed] [Google Scholar]
- Kolář F., Štech M., Trávníček P., Rauchová J., Urfus T., Vít P., et al. (2009). Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Ann. Bot. 103 963–974. 10.1093/aob/mcp016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron P., Suda J., Husband B. C. (2007). Applications of flow cytometry to evolutionary and population biology. Annu. Rev. Ecol. Evol. Syst. 38 847–876. 10.1146/annurev.ecolsys.38.091206.095504 [DOI] [Google Scholar]
- Laere V. K., França S. C., Vansteenkiste H., Van Huylenbroeck J., Steppe K., Van Labeke M. C. (2011). Influence of ploidy level on morphology, growth and drought susceptibility in Spathiphyllum wallisii. Acta Physiol. Plant. 33 1149–1156. 10.1007/s11738-010-0643-2 [DOI] [Google Scholar]
- Lago Canzobre E., Castroviejo S. (1992). Estudio Citotaxonómico de la Flora de las Costas Gallegas. Cadernos Área Ciencias Biolóxicas 3. Publicacións do Seminario de Estudos Galegos. Sada, A Coruña: Edicións do Castro. [Google Scholar]
- Laport R. G., Hatem L., Minckley R. L., Ramsey J. (2013). Ecological niche modeling implicates climatic adaptation, competitive exclusion, and niche conservatism among Larrea tridentata cytotypes in North American deserts1, 2. J. Torrey Bot. Soc. 140 349–364. 10.3159/TORREY-D-13-00009.1 [DOI] [Google Scholar]
- Laport R. G., Ng J. (2017). Out of one, many: the biodiversity considerations of polyploidy. Am. J. Bot. 104 119–1121. 10.3732/ajb.1700190 [DOI] [PubMed] [Google Scholar]
- Leitão M. T., Paiva J. A. (1988). El endemismo lusitano de Jasione L. (Campanulaceae). Lagascalia 15 341–344. [Google Scholar]
- Levin D. A. (1975). Minority cytotype exclusion in local plant populations. Taxon 24 35–43. 10.2307/1218997 [DOI] [Google Scholar]
- Levin D. A. (2002). The Role of Chromosomal Change in Plant Evolution. Oxford: Oxford University Press. [Google Scholar]
- Levin D. A. (2003). The cytoplasmic factor in plant speciation. Syst. Bot. 28 5–11. [Google Scholar]
- Lexer C., van Loo M. (2006). Contact zones: natural labs for studying evolutionary transitions. Curr. Biol. 16 R407–R409. 10.1016/j.cub.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Li B. H., Xu X. M., Ridout M. S. (2004). Modelling the establishment and spread of autotetraploid plants in a spatially heterogeneous environment. J. Evol. Biol. 17 562–573. 10.1111/j.1420-9101.2004.00700.x [DOI] [PubMed] [Google Scholar]
- Li W. L., Berlyn G. P., Ashton P. M. S. (1996). Polyploids and their structural and physiological characteristics relative to water deficit in Betula papyrifera (Betulaceae). Am. J. Bot. 83 15–20. 10.1002/j.1537-2197.1996.tb13869.x [DOI] [Google Scholar]
- López-Jurado J., Mateos-Naranjo E., Balao F. (2019). Niche divergence and limits to expansion in the high polyploid Dianthus broteri complex. New Phytol. 222 1076–1087. 10.1111/nph.15663 [DOI] [PubMed] [Google Scholar]
- Loureiro J., Rodriguez E., Doležel J., Santos C. (2007). Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann. Bot. 100 875–888. 10.1093/aob/mcm152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceira N. O., Jacquard P., Lumaret R. (1993). Competition between diploid and derivative autotetraploid Dactylis glomerata L. from Galicia. Implications for the establishment of novel polyploid populations. New Phytol. 124 321–328. 10.1111/j.1469-8137.1993.tb03822.x [DOI] [PubMed] [Google Scholar]
- Maherali H., Walden A. E., Husband B. C. (2009). Genome duplication and the evolution of physiological responses to water stress. New Phytol. 184 721–731. 10.1111/j.1469-8137.2009.02997.x [DOI] [PubMed] [Google Scholar]
- Manzaneda A. J., Rey P. J., Anderson J. T., Raskin E., Weiss-Lehman C., Mitchell-Olds T. (2015). Natural variation, differentiation, and genetic trade-offs of ecophysiological traits in response to water limitation in B. distachyon and its descendent allotetraploid B. hybridum (Poaceae). Evolution 69 2689–2704. 10.1111/evo.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzaneda A. J., Rey P. J., Bastida J. M., Weiss-Lehman C., Raskin E., Mitchell-Olds T. (2012). Environmental aridity is associated with cytotype segregation and polyploidy occurrence in Brachypodium distachyon (Poaceae). New Phytol. 193 797–805. 10.1111/j.1469-8137.2011.03988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant D. B., Soltis D. E., Soltis P. S. (2016). Patterns of abiotic niche shifts in allopolyploids relative to their progenitors. New Phytol. 212 708–718. 10.1111/nph.14069 [DOI] [PubMed] [Google Scholar]
- Marques I., Loureiro J., Draper D., Castro M., Castro S. (2017). How much do we know about the frequency of hybridization and polyploidy in the Mediterranean region? Plant Biol. 20 21–37. 10.1111/plb.12639 [DOI] [PubMed] [Google Scholar]
- Martin S. L., Husband B. C. (2013). Adaptation of diploid and tetraploid Chamerion angustifolium to elevation but not local environment. Evolution 67 1780–1791. 10.1111/evo.12065 [DOI] [PubMed] [Google Scholar]
- Masterson J. (1994). Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264 421–424. 10.1126/science.264.5157.421 [DOI] [PubMed] [Google Scholar]
- Molina-Henao Y. F., Hopkins R. (2019). Autopolyploid lineage shows climatic niche expansion but not divergence in Arabidopsis arenosa. Am. J. Bot. 106 61–70. 10.1002/ajb2.1212 [DOI] [PubMed] [Google Scholar]
- Muñoz-Pajares A. J., Perfectti F., Loureiro J., Abdelaziz M., Biella P., Castro M., et al. (2018). Niche differences may explain the geographic distribution of cytotypes in Erysimum mediohispanicum. Plant Biol. 20 139–147. 10.1111/plb.12605 [DOI] [PubMed] [Google Scholar]
- Nassar N. M. A., Graciano-Ribeiro D., Fernandes S. D. C., Araújo P. C. (2008). Anatomical alterations due to polyploidy in cassava, Manihot esculenta Crantz. Genet. Mol. Res. 7 276–283. 10.4238/vol7-2gmr399 [DOI] [PubMed] [Google Scholar]
- Nuismer S. L., Cunningham B. M. (2005). Selection for phenotypic divergence between diploid and autotetraploid Heuchera grossulariifolia. Evolution 59 1928–1935. 10.1111/j.0014-3820.2005.tb01062.x [DOI] [PubMed] [Google Scholar]
- Otto S. P., Whitton J. (2000). Polyploid incidence and evolution. Annu. Rev. Genet. 34 401–437. 10.1146/annurev.genet.34.1.401 [DOI] [PubMed] [Google Scholar]
- Parisod C., Broennimann O. (2016). Towards unified hypotheses of the impact of polyploidy on ecological niches. New Phytol. 212 540–542. 10.1111/nph.14133 [DOI] [PubMed] [Google Scholar]
- Parnell J. A. (1980). The Experimental Taxonomy of Jasione montana L. Ph.D dissertation, University of Aberdeen, Aberdeen. [Google Scholar]
- Parnell J. A. (1982). Some observations on the breeding biology of Jasione montana L. J. Life Sci. 4 1–7. [Google Scholar]
- Parnell J. A. (1985). Jasione montana L. J. Ecol. 73 341–358. 10.2307/2259787 30510465 [DOI] [Google Scholar]
- Parnell J. A. (1987). Variation in Jasione montana L. (Campanulaceae) and related species in Europe and North Africa. Watsonia 16 249–267. [Google Scholar]
- Peco B., Lopez-Merino L., Alvir M. (2006). Survival and germination of Mediterranean grassland species after simulated sheep ingestion: ecological correlates with seed traits. Acta Oecol. 30 269–275. 10.1016/j.actao.2006.05.004 [DOI] [Google Scholar]
- Pérez-Espona S., Sales F., Hedge I., Möller M. (2005). Phylogeny and species relationships in Jasione (Campanulaceae) with emphasis on the ‘Montana-complex’. Edinb. J. Bot. 62 29–51. 10.1017/S0960428606000047 [DOI] [Google Scholar]
- Petit C., Bretagnolle F., Felber F. (1999). Evolutionary consequences of diploid–polyploid hybrid zones in wild species. Trends Ecol. Evol. 14 306–311. 10.1016/s0169-5347(99)01608-0 [DOI] [PubMed] [Google Scholar]
- Phillips S. J. (2008). Transferability, sample selection bias and background data in presence-only modelling: a response to Peterson et al., (2007). Ecography 31 272–278. 10.1111/j.0906-7590.2008.5378.x [DOI] [Google Scholar]
- Phillips S. J., Anderson R. P., Schapire R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- Praeger R. L. (1913). On the buoyancy of the seeds of some Britannic plants. Sci. Proc. R. Dublin Soc. 14 13–62. [Google Scholar]
- R Core Development Team (2016). A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ramsey J. (2011). Polyploidy and ecological adaptation in wild yarrow. Proc. Natl. Acad. Sci. U.S.A. 108 7096–7101. 10.1073/pnas.1016631108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J., Ramsey T. (2014). Ecological studies of polyploidy in the 100 years following its discovery. Philos. Trans. R. Soc. B Biol. Sci. 369:20130352. 10.1098/rstb.2013.0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J., Schemske D. W. (2002). Neopolyploidy in flowering plants. Annu. Rev. Ecol. Evol. Syst. 33 589–639. 10.1146/annurev.ecolsys.33.010802.150437 [DOI] [Google Scholar]
- Rivas-Martínez S., Penas A., Díaz T. E., Cantó P., del Río S., Costa J. C., et al. (2017). “Iogeographic units of the Iberian Peninsula and Islas Baleares to district level. A concise synopsis,” in The Vegetation of the Iberian Peninsula 1, ed. Loidi J. (Cham: Springer; ), 131–188. 10.1007/978-3-319-54784-8_5 [DOI] [Google Scholar]
- Rodríguez D. J. (1996). A model for the establishment of polyploidy in plants. Am. Nat. 147 33–46. 10.1086/285838 [DOI] [Google Scholar]
- Rubido-Bará M., Horjales Luaces M., Villaverde C. (2010). Dos nuevas subespecies del género Jasione L. (Campanulaceae) en el Noroeste de la Península Ibérica. Nova Acta Cient. Compostel. Biol. 19 21–31. [Google Scholar]
- Sales F., Hedge I. C. (2001). “Flora iberica. plantas vasculares de la Península ibérica e islas baleares” in Myoporaceae-Campanulaceae, Vol. XIV, eds Castroviejo S., Paiva J., Hedge I. C., Aedo C., Aldasoro J. J., et al. (Madrid: Real Jardin Botanico, CSIC; ). [Google Scholar]
- Sales F., Hedge I. C., Eddie W., Preston J., Moeller M. (2004). Jasione L. taxonomy and phylogeny. Turk. J. Bot. 28 253–259. [Google Scholar]
- Segraves K. A., Anneberg T. J. (2016). Species interactions and plant polyploidy. Am. J. Bot. 103 1326–1335. 10.3732/ajb.1500529 [DOI] [PubMed] [Google Scholar]
- Segraves K. A., Thompson J. N. (1999). Plant polyploidy and pollination: floral traits and insect visits to diploid and tetraploid Heuchera grossulariifolia. Evolution 53 1114–1127. 10.1111/j.1558-5646.1999.tb04526.x [DOI] [PubMed] [Google Scholar]
- Siopa C., Dias M. C., Castro M., Loureiro J., Castro S. (2020). Is selfing a reproductive assurance promoting polyploid establishment? Reduced fitness, leaky self-incompatibility and lower inbreeding depression in Neotetraploids. Am. J. Bot. 10.1002/ajb2.1441 [DOI] [PubMed] [Google Scholar]
- Soltis D. E., Buggs R. J., Doyle J. J., Soltis P. S. (2010). What we still don’t know about polyploidy. Taxon 59 1387–1403. 10.1002/tax.595006 [DOI] [Google Scholar]
- Soltis D. E., Soltis P. S. (1999). Polyploidy: recurrent formation and genome evolution. Trends Ecol. Evol. 14 348–352. 10.1016/s0169-5347(99)01638-9 [DOI] [PubMed] [Google Scholar]
- Soltis D. E., Soltis P. S., Schemske D. W., Hancock J. F., Thompson J. N., Husband B. C., et al. (2007). Autopolyploidy in angiosperms: have we grossly underestimated the number of species? Taxon 56 13–30. 10.2307/25065732 [DOI] [Google Scholar]
- Sonnleitner M., Flatscher R., Escobar García P., Rauchová J., Suda J., Schneeweiss G. M., et al. (2010). Distribution and habitat segregation on different spatial scales among diploid, tetraploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps. Ann. Bot. 106 967–977. 10.1093/aob/mcq192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelhof J. P., Soltis P. S., Soltis D. E. (2017). Pure polyploidy: closing the gaps in autopolyploid research. J. Syst. Evol. 55 340–352. 10.1111/jse.12253 [DOI] [Google Scholar]
- Stebbins G. L. (1938). Cytological characteristics associated with the different growth habits in the dicotyledons. Am. J. Bot. 25 189–198. 10.1002/j.1537-2197.1938.tb09203.x [DOI] [Google Scholar]
- Stebbins G. L. (1950). Variation and Evolution in Plants. New York, NY: Columbia University Press, 10.7312/steb94536 [DOI] [Google Scholar]
- Stebbins G. L. (1971). Chromosome Evolution in Higher Plants. New York, NY: Columbia University Press. [Google Scholar]
- Suda J., Kron P., Husband B. C., Trávníček P. (2007). “Flow cytometry and ploidy: applications in plant systematics, ecology and evolutionary biology,” in Flow Cytometry With Plant Cells: Analysis of Genes, Chromosomes and Genomes, eds Dolezel J., Greilhuber J., Suda J. (Weinheim: Wiley-VCH; ), 103–130. 10.1002/9783527610921.ch5 [DOI] [Google Scholar]
- Thiers B. (2017). continuously Updated. Index Herbariorum: a Global Directory of Public Herbaria and Associated Staff. New York, NY: New York Botanical Garden’s. [Google Scholar]
- Thompson K. A., Husband B. C., Maherali H. (2014). Climatic niche differences between diploid and tetraploid cytotypes of Chamerion angustifolium (Onagraceae). Am. J. Bot. 101 1868–1875. 10.3732/ajb.1400184 [DOI] [PubMed] [Google Scholar]
- Thompson K. A., Husband B. C., Maherali H. (2015). No influence of water limitation on the outcome of competition between diploid and tetraploid Chamerion angustifolium (Onagraceae). J. Ecol. 103 733–741. 10.1111/1365-2745.12384 [DOI] [Google Scholar]
- Thuiller W., Georges D., Engler R., Breiner F., Georges M. D., Thuiller C. W. (2016). Package ‘biomod2’. Available online at: https://cran.r-project.org/package=biomod2 (accessed February 2018). [Google Scholar]
- Tutin T. G., Heywood V. H., Burges N. A., Valentine D. H. (1976). Flora Europaea: Plantaginaceae to Compositae (and Rubiaceae), Vol. 4 Cambridge: Cambridge University Press. [Google Scholar]
- Visger C. J., Germain-Aubrey C. C., Patel M., Sessa E. B., Soltis P. S., Soltis D. E. (2016). Niche divergence between diploid and autotetraploid Tolmiea. Am. J. Bot. 103 1396–1406. 10.3732/ajb.1600130 [DOI] [PubMed] [Google Scholar]
- Warren D. L., Glor R. E., Turelli M. (2008). Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62 2868–2883. 10.1111/j.1558-5646.2008.00482.x [DOI] [PubMed] [Google Scholar]
- Warren D. L., Glor R. E., Turelli M. (2010). ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33 607–611. 10.1111/j.1600-0587.2009.06142.x [DOI] [Google Scholar]
- Wefferling K. M., Castro S., Loureiro J., Castro M., Tavares D., Hoot S. B. (2017). Cytogeography of the subalpine marsh marigold polyploid complex (Caltha leptosepala sl, Ranunculaceae). Am. J. Bot. 104 271–285. 10.3732/ajb.1600365 [DOI] [PubMed] [Google Scholar]
- Wood T. E., Takebayashi N., Barker M. S., Mayrose I., Greenspoon P. B., Rieseberg L. H. (2009). The frequency of polyploid speciation in vascular plants. Proc. Natl. Acad. Sci. U.S.A. 106 13875–13879. 10.1073/pnas.0811575106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Principal component analyses: (A) for the entire geographic distribution of J. maritima (in Portugal, Spain and France), using 19 bioclimatic variables (Bio1-Bio19) plus elevation extracted for all species occurrences from the Worldclim database at a resolution of 1 km; (B) for the contact zone (in the northwest Iberian Peninsula), using the following set of variables at 100 m resolution – elevation (ele); aspect, slope, slope range (slprng) and topographic position index (tpi); summer mean temperature (tmed) and mean annual precipitation (pp); lithology (lito); and distance to coast (dist_coast). Variance explained by each axis is also provided.
Geographic information of the Jasione maritima populations sampled in this study for flow cytometric analyses. For each population, an ID code, locality name, herbarium code at SANT, geographical coordinates (angular), sample size (N, number of individuals analyzed) and estimated DNA ploidy level are given. Ploidy levels: diploids (2x) and tetraploids (4x). The two ID codes marked with an asterisk (∗) denote populations where seeds were collected for chromosome counts.
Environmental variables characterization in Jasione maritima considering the total distribution area. For each environmental variable mean and standard error of the mean (se) per cytotype are presented. F and P values are also presented. Different letter corresponds to statistically differences (P < 0.05) between the groups for a given environmental variable. Shades highlight differences between cytotypes/varieties. Bold highlight variables used in niche modeling.
Environmental variables characterization in Jasione maritima in the contact zone (northwest of Iberian Peninsula) area. For each variable mean and standard error of the mean (se) are presented. F and P values are also presented. Different letter corresponds to statistically differences (P < 0.05) between the group within the same environmental variable. Shades highlight differences between cytotypes/varieties. Bold highlight variables used in niche modeling.
Variable contribution in Principal Component Analyses using all variables (Figure 1) at total distribution area and contact zone analyses.
Jasione maritima models’ evaluation. For diploid individuals of J. maritima var. maritima (2x var. maritima), tetraploid individuals of J. maritima var. maritima (4x var. maritima) and tetraploid individuals of J. maritima var. sabularia (4x var. sabularia) mean and standard error of AUC values of the models and of omission rates were presented for each cytotype in the different approaches. The value of omission rate of the final model for each cytotype/approach were also presented.
Genome size estimates in Jasione maritima. In each population, DNA ploidy estimates and mean, standard deviation of the mean (SD), coefficient of variation (CV, in %), minimum (Min) and maximum (Max) values of holoploid genome size (2C, in pg) are given. Number of individuals analyzed for genome size in each population (n) and mean monoploid genome size (1Cx, in pg) are also provided. Ploidy levels: diploids (2x) and tetraploids (4x).
Jasione maritima environmental niches overlapping comparing the two varieties and two cytotypes in pairs (diploid niche of J. maritima var. maritima – 2x var. maritima, tetraploid niche of J. maritima var. maritima – 4x var. maritima and tetraploid niche of J. maritima var. sabularia – 4x var. sabularia). The percentage of variance explained the two-principal axis, the total of variance explained, percentage of environmental niche overlapping of cytotype A niche in cytotype B niche (A → B) and cytotype B niche in cytotype A niche (B → A) were present for each comparison in the two approaches.
Variable contribution in Principal Component Analyses using selected variables using in the models (Figure 5) at total distribution area and contact zone analyses.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.