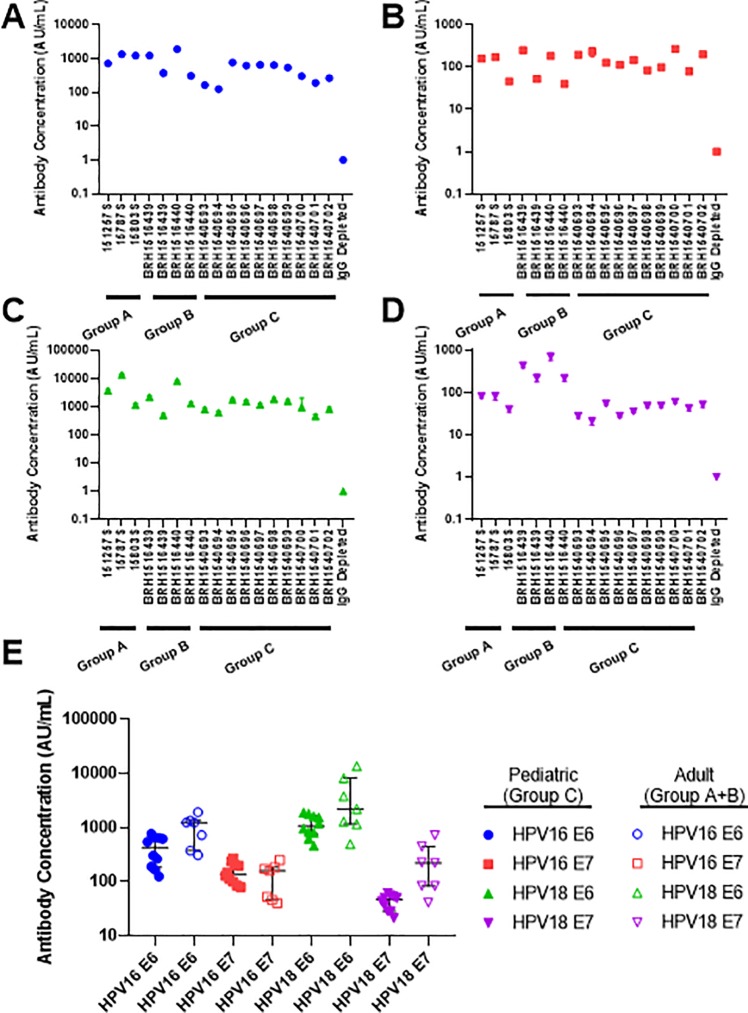
Fig 4. Reproducibility and validation of the HPV multiplex MSD-based serology assay for various donor groups.
Group A–HPV+ cervical cancer subjects (n = 3); Group B–normal, healthy adult donors (n = 4); Group C–normal pediatric donors (n = 10). A–inter-subject variability for evaluating the concentration of IgG anti-HPV16 E6 over multiple assays (n = 5 assays). B–inter-subject variability for evaluating the concentration of IgG anti-HPV16 E7 over multiple assays (n = 6 assays). C—inter-subject variability for evaluating the concentration of IgG anti-HPV18 E6 over multiple assays (n = 5 assays). D—inter-subject variability for evaluating the concentration of IgG anti-HPV18 E7 over multiple assays (n = 5 assays). E–Inter-group anti-HPV IgG concentrations plotted as median ± interquartile range. Open shapes are Groups A and B and filled shapes is Group C.