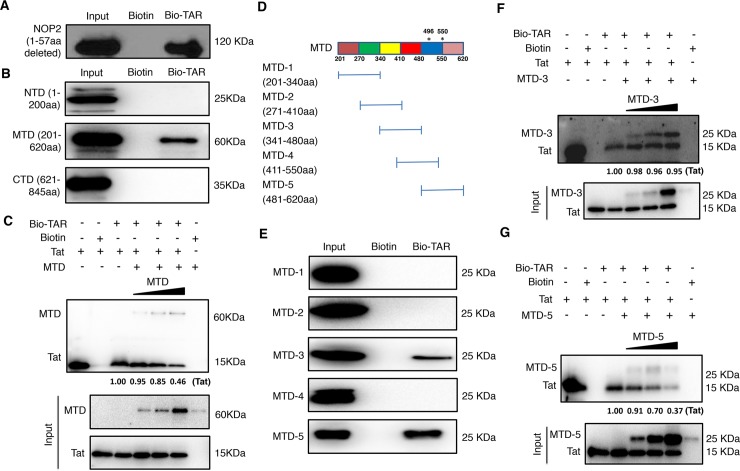
Fig 6. The MTD domain of NOP2 competes with Tat for TAR binding.
(A) Recombinant NOP2 protein with deletion of 1–57 amino acids (6xHis-tagged) was purified from bacteria, and incubated with bio-TAR or free biotin in vitro. The protein-RNA complex was affinity-precipitated using streptavidin magnetic beads. The input or precipitated NOP2 (1-57aa deleted) protein was analyzed by immunoblotting. (B) Recombinant NOP2 protein domains, NTD (1-200aa), MTD (201-620aa), and CTD (621-845aa), were purified from bacteria, and incubated with bio-TAR or free biotin in vitro, followed by the affinity precipitation using streptavidin magnetic beads. The input or precipitated NOP2 protein domains (NTD, MTD, CTD) were analyzed by immunoblotting. (C) Recombinant Tat protein was incubated with bio-TAR in the absence or presence of NOP2 MTD domain at the increased doses, or with free biotin in vitro, followed by the affinity precipitation using streptavidin magnetic beads. The input or precipitated Tat or NOP2 MTD domain was analyzed by immunoblotting. The relative intensity of pulled down Tat was calculated. (D) NOP2 MTD domain was further divided into five smaller domains (MTD-1 to 5), which were ~140aa with 70aa overlap. The positions of two catalytic cysteine residues (496aa, 550aa) in the MTD were indicated by asterisks (*). (E) Recombinant NOP2 MTD smaller domains, MTD-1 to 5, were purified from bacteria, and incubated with bio-TAR or free biotin in vitro, followed by the affinity precipitation using streptavidin magnetic beads. The input or precipitated smaller MTD domains of NOP2 were analyzed by immunoblotting. (F, G) Recombinant Tat protein was incubated with bio-TAR in the absence or presence of NOP2 MTD-3 (F) or MTD-5 (G) domain at the increased amount, or with free biotin in vitro, followed by the affinity precipitation using streptavidin magnetic beads. The input or precipitated Tat or smaller MTD domain of NOP2 was analyzed by immunoblotting. The relative intensity of pulled down Tat was calculated.