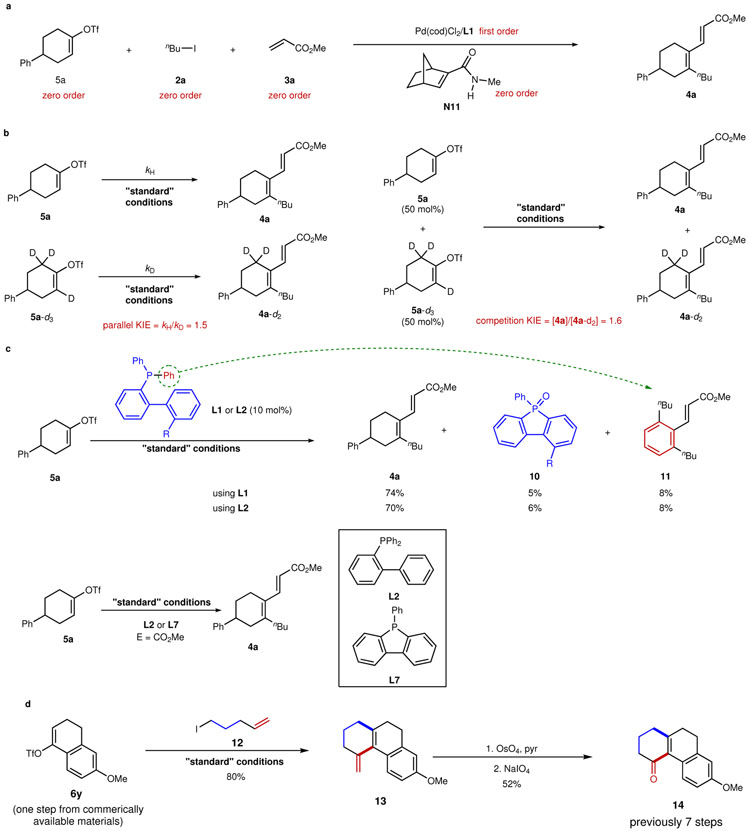
Figure 3∣. Mechanistic studies and synthetic utility.
a, Determination of the reaction order: zero-order kinetics for 5a, 2a, 3a and N11, and first-order kinetics for [Pd/L1] were observed, indicating that oxidative addition of 5a, migratory insertion into N11, the reaction with 2a, and migratory insertion into 3a are not the turnover-limiting step. b, The parallel and competition kinetic isotopic effects (KIE) were measured, indicating that the C–H cleavage step is only partially turnover-limiting. c, The observation of cyclized phosphafluorene oxide 10 and by-product 11, together with the parallel kinetic study between L2 and L7, indicate that the actual ligand in this system is likely the corresponding phosphafluorene. d. Synthesis of tricyclic compound 14 is illustrated using this method, which uses fewer steps than the prior route.