Table 1.
NBE Effect for the Alkenyl Catellani Reactiona
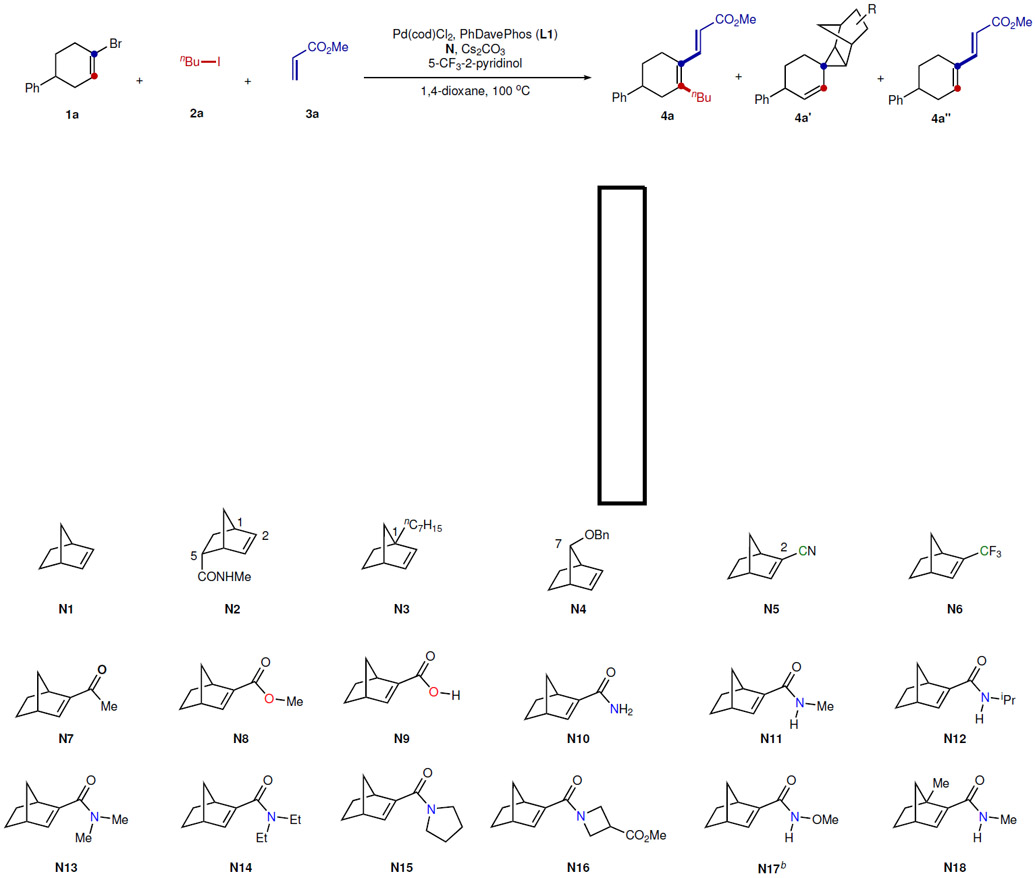 |
Reaction conditions: 1a (0.10 mmol), 2a (0.30 mmol), 3a (0.15 mmol), Pd(cod)Cl2 (0.01 mmol), L1 (0.01 mmol), N (0.15 mmol), 5-trifluomethyl-2-pyridinol (0.02 mmol), Cs2CO3 (0.30 mmol), 100 °C, 16 h. Yield determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard.
The conversion was 19%.
