Table 3.
Reaction scope
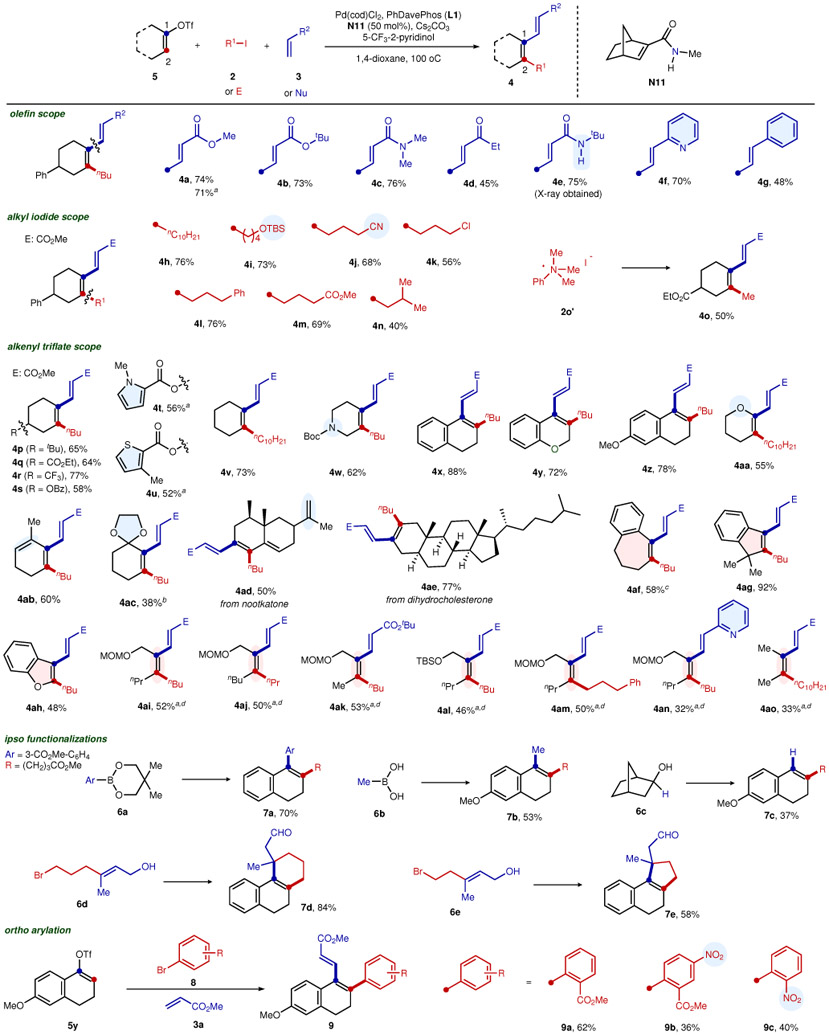 |
*Reaction conditions: 5 (0.30 mmol), 2 (0.90 mmol), 3 (0.45 mmol), Pd(cod)Cl2 (0.03 mmol), L1 (0.03 mmol), N11 (0.15 mmol), 5-trifluomethyl-2-pyridinol (0.06 mmol), Cs2CO3 (0.90 mmol), 1,4-dioxane (6 mL), 100 °C, 16 h.
The corresponding alkenyl bromides were used instead of 5.
The corresponding alkenyl iodide was used instead of 5.
L2 (0.03 mmol) was used instead of L1.
N11 (0.30 mmol) was used, K3PO4 (0.90 mmol) was used instead of Cs2CO3, and a mixed solvent of 1,4-dioxane (3 mL) and toluene (3 mL) was used instead of 1,4-dioxane alone.
