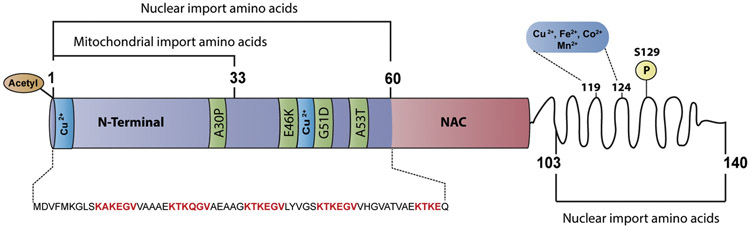
Figure 1. Structural domain map of α-synuclein.
Schematic representation of α-synuclein structural domains indicating the potential residues involved in nuclear and mitochondrial localization. Residues 1-60 and 103-140 are involved in the nuclear localization of α-synuclein (Ma et al., 2014). Residues 1-33 potentially contain a cryptic mitochondrial targeting signal (Devi et al., 2008). Missense α-synuclein mutations, including A30P, which is associated with familial PD, occur in the N-terminal domain. The aggregation-prone non-amyloidogenic region (NAC) is part of the membrane-binding domain. The C-terminal domain is proposed to be disordered and modulates interactions with various molecules including pro-oxidant metals associated with PD pathology.