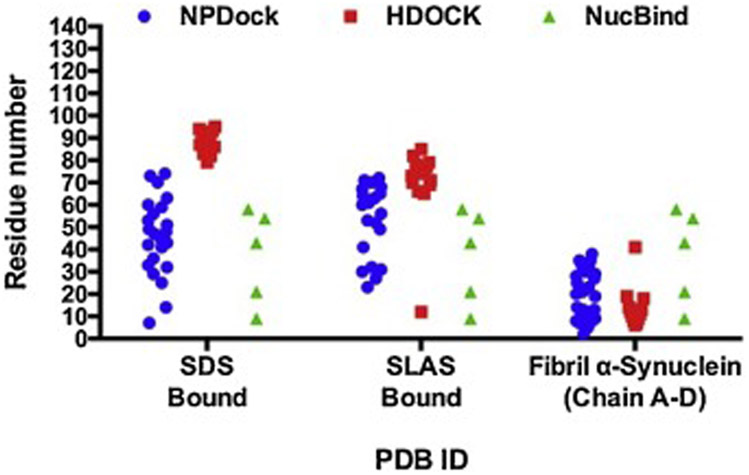
Figure 4. Comparative analysis of two DNA-protein docking methods for three characterized PDB structures of α-synuclein.
Amino acid residues 60-100 within the NAC domain are the most likely to interact with DNA when α-synuclein adopts an α-helical folded conformation (PDB ID: 1XQ8 and 2KKW). Amino acids residues located in either the N-terminal or the C-terminal of the α-synuclein fibril structure (PDB ID: 2N0A) are more free to interact with DNA.