Developing any diagnostic assay that receives United States Food and Drug Administration (FDA) approval can be a slow and difficult process. FDA-approved assays for fungal diagnosis are generally few in number and are focused mainly on diagnosing candidiasis, which is caused by several species of Candida, in addition to a limited number of systemic mycotic agents. While all microbial diagnostic assays face challenges before they are FDA approved and reach the market, there are a number of challenges to fungal diagnostic assay development that have been difficult hurdles to overcome.
KEYWORDS: antifungal, molds, mycoses, nomenclature, susceptibility testing, yeast
ABSTRACT
Developing any diagnostic assay that receives United States Food and Drug Administration (FDA) approval can be a slow and difficult process. FDA-approved assays for fungal diagnosis are generally few in number and are focused mainly on diagnosing candidiasis, which is caused by several species of Candida, in addition to a limited number of systemic mycotic agents. While all microbial diagnostic assays face challenges before they are FDA approved and reach the market, there are a number of challenges to fungal diagnostic assay development that have been difficult hurdles to overcome. These hurdles include template preparation, fungal morphology, how many fungi should be identified in a single assay (scope), taxonomy and nomenclature, discriminating colonizers from invasive infection, combining identification with antifungal susceptibility, and navigating the administrative hurdles required to integrate an assay into a clinical laboratory. Some of these challenges are easier to overcome than others, but all seem to be particularly difficult for fungal diagnostic assays.
INTRODUCTION
The challenges facing diagnostic microbiology, and medical mycology in particular, continue to grow in spite of newer technologies being employed to develop diagnostic assays. Multiple factors have contributed to increase the number and difficulty of challenges to the clinical microbiologist charged with identifying fungal pathogens from patients. Most of the current challenges to the microbiology laboratory are due to new populations of patients that are more immunocompromised for a longer period of time during the course of their illness. These patients are increasingly predisposed to becoming infected by a wider variety of fungi, which remain major causes of morbidity and mortality. In spite of new diagnostic technologies entering the clinical microbiology laboratory, they have been slow to be approved for diagnosis of fungi. This deficiency has contributed to delayed diagnosis, which is a known risk factor for increased morbidity and mortality in many systemic mycoses (1).
Part of the delayed diagnosis problem in clinical mycology is the diverse and growing spectrum of fungi capable of causing disease. Many of these emerging fungal pathogens have not been seen at a high enough frequency to be considered early on in the course of infection, and in many cases, if a fungus is determined to be the etiologic agent, it often cannot be identified to the species level. Consequently, rarely seen or unusual fungi often need to be sent out to reference laboratories for proper identification. In fact, the Centers for Disease Control and Prevention has played a crucial role for multiple identifications of rare fungal pathogens within just the last few years (2–4). Other fungi, such as the recently emergent Candida auris, are close relatives of more commonly seen fungi and may not be discriminated from these more common relatives (5). In fact, emergent mycoses have proven to pose consistent diagnostic challenges for many decades, due in part to changing patient susceptibilities.
In this review, we summarize some of the more problematic challenges to developing new molecular diagnostic assays. We have focused on molecular assays that target nucleic acids and proteins but recognize that assays directed toward metabolites, antigens, or antibodies have their own challenges. Still, other assays that are more traditional such as biochemical, serological, or morphological also have challenges and have been reviewed elsewhere (6–9). Our perspective is from both a basic research view, which in and of itself is often not encumbered by how difficult or expensive a new and sensitive technique could be to implement in a clinical microbiology laboratory, and a clinical perspective, which has to balance cost, ease of use, and consistency with how sensitive and accurate an assay could be in the real world of a clinical microbiology laboratory. There are numerous critical and even required characteristics of an assay that do not need to be elaborated on here since they are goals of virtually any assay and remain important challenges to any assay development. These goals include high precision and accuracy, wide reportable range, high analytical sensitivity, rapid turnaround, instrument and assay cost, instrument footprint, and high positive and negative predictive values. For fungi, there are additional challenges, which we feel are extreme and need to be considered for any new fungal diagnostic.
TEMPLATE PREPARATION
In spite of a diverse approach to fungal diagnostic assay development, one of the most frequently overlooked components of any assay is template or target preparation. Fungi are unusual compared to other microbes because of their rigid cell walls, which necessitate preliminary preparation steps that require removal during the sample preparation process. These steps can be chemical, physical, or enzymatic, and each of these strategies has numerous methods. Pure cultures are much easier to process because 100% of the assay target will be fungal derived, and there generally is no limitation on the starting material amount. However, the best assays work directly on human specimens with no need for fungal outgrowth in pure culture. This requirement brings multiple problems. First, in general, fungal elements will typically be few in number in tissue or body fluids and can be vastly overwhelmed by host material, which can include whole tissue, blood, sputum, and other body fluids, each of which may bring specific problems to the preparation. For example, hyphae from members of the Mucorales are more delicate than other filamentous fungi and can be destroyed by tissue preparations that utilize grinding or other physical methods, which can result in erroneously low or even absent CFU values. Molds in general, in contrast to yeasts, can be challenging to precisely enumerate because a single hyphal fragment, which may contain many nuclei, can grow as a single CFU after plating but yield multiple CFU by PCR. Second, fungal cell wall material often needs to be removed at some point during the extraction process since it can inhibit downstream reactions. Melanin, a polyphenolic compound found in many fungi, for example, is a known inhibitor of PCR (10). Polysaccharides, which many fungi produce in abundance, can also inhibit PCR (11). Because polysaccharides can be purified along with DNA if not removed in a separate step, a common chemical used in fungal nucleic acid extraction is CTAB (cetyltrimethylammonium bromide), which is a cationic detergent that is used to remove polysaccharides during DNA purification. However, incorporating this reagent into a protocol adds another step and additional tube manipulations. Third, if the assay employs a PCR step, the specific extraction protocol or device must be free of contamination, including associated reagents, which requires great care to accomplish reliably. Fungal nucleic acids have been found to contaminate primers, probes, and master mix solutions (12, 13). Furthermore, because of the sensitivity of PCR and the possibility of amplifying contaminating DNA from any source, the various aspects of these assays, including template preparation, PCR, and other downstream steps, may need to be performed in separate areas and within containment hoods that protect samples from becoming contaminated with aerosolized amplicons. While preventing contamination for any microbe-specific PCR assay is crucial, fungi can be especially challenging because many contaminating ubiquitous molds common in the environment can also be human pathogens that could potentially be present in a clinical specimen.
Because clinical laboratories rely on standardized methods and are operated under certification from a variety of entities, some automated extraction instruments have been developed that work quite well. Most of these are magnetic bead-based instruments (14) and may need a preprocessing step that removes the fungal cell wall prior to the sample being loaded into the instrument. However, in the case of DNA, the template can be purified to a very high level and with high efficiency. Unfortunately, because extraction is such a challenging problem for fungal diagnosis, many procedures or systems are independent of the actual assay and need to be purchased or developed separately from the specific assay. One assay that incorporates sample preparation into the assay is the T2Dx instrument (T2 Biosystems, Lexington, MA), which is FDA approved for diagnosing Candida sp. infections from blood specimens. This system is an example of a “load and walk away” diagnostic system, where the specimen is loaded into the instrument with little preprocessing and data are collected at the end of the run leading to an identification. These are ideal assays for a clinical microbiology laboratory because they require only minimal technical skills and little sample manipulation or interpretation. However, instruments that incorporate their own extraction modules may be easier to develop for yeast pathogens since this morphology is much easier to extract assay targets from than molds. While not universally applicable, target extraction from yeast cells can sometimes be done without physical breakage in contrast to hyphae, which almost always need a physical breakage step. These differences can be difficult to address with a one-size-fits-all instrument.
ACCOUNTING FOR FUNGAL MORPHOLOGIC VARIATION
While not unique in microbiology, fungi probably display more morphological variation and to a larger degree than most microbes. In tissue, they can grow as yeast, which can be similar to a typical bacterial cell in consistency, or mold, which is filamentous and hyphal in nature. A smaller subset, the dimorphic fungi, which includes many of the major systemic mycoses, can grow in both morphologies, although few exhibit both in vivo (Fig. 1). In vitro morphologies can be even more varied, especially for the molds as sporulation is more common than that in vivo. For assays that rely on cell lysates to release a target, morphology is less of a problem, although it often can require different strategies for cell lysis and different degrees of cleanup after lysis to remove cellular material that is not targeted by the assay. For all morphologies, lysing cells becomes harder as the cultures age and typically yields better results on fresh or logarithmic phase cultures, which can add additional delay if a subculture must be made from the primary specimen.
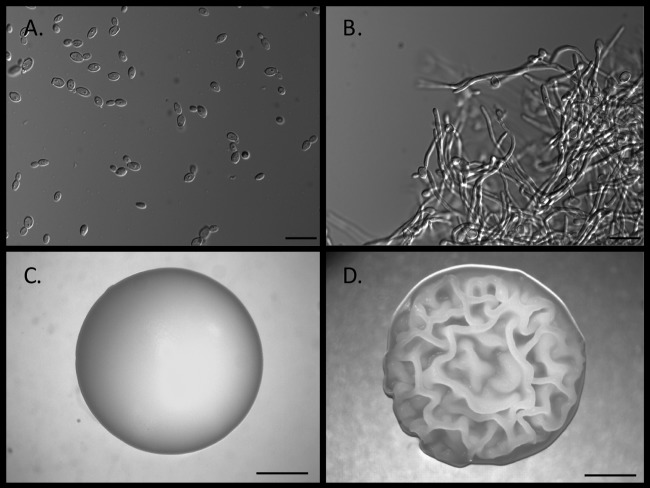
FIG 1.
Dimorphism in Candida albicans. C. albicans is an example of a dimorphic fungus. These fungi can grow as budding yeast or true septate hyphae. C. albicans also can produce pseudohyphae and, depending on culture conditions and duration, chlamydospores. Depending on the type of assay, morphology can affect the outcome or it can determine whether the culture can even be run on a specific assay or instrument. (A) C. albicans yeast cells grown in broth culture for 20 h. Bar = 10 μm. (B) C. albicans grown in broth culture showing predominantly hyphal growth. Bar = 10 μm. (C) C. albicans growing on agar medium as a typical yeast colony with a creamy texture and smooth surface. Bar = 1.0 cm. (D) C. albicans growing on agar medium showing a rough colony morphology that has hyphae growing under the colony. Bar = 1.0 cm. (Images courtesy of David Kadosh, reproduced with permission.)
Whole-cell assays can be very problematic. Yeast cells, because of their unicellular nature, are easier to manage because they are smaller, easier to quantify by direct counting or optical density (OD), more uniform in size, and usually not as rigid as hyphae. Yeast cells also typically have a single nucleus per cell. A filamentous morphology, conversely, can result in hyphal or pseudohyphal filaments that vary in length; spores that also can vary in size, shape, and hydrophobicity; and other structures that may grow off hyphae. Hyphae can also be multinucleate, making quantification more difficult compared to that for a yeast cell. A single nucleus per cell allows a more precise determination of CFU when trying to quantitate, such as by real-time PCR, which is very difficult to do when working with a hyphal culture. The filamentous morphology inhibits a precise cell count in liquid cultures, which may be needed prior to running an assay because broth cultures of filamentous cells can result in growth as fungal balls, a single clot of hyphae, or numerous clusters, which makes normalizing for cell number extremely difficult or impossible. Agar cultures of hyphae need to be scraped, which still presents problems, as hyphae will be removed as fragments in addition to potential contaminating pieces of agar. If the culture is a sterile mold, which produces few if any differentiating structures, it cannot be identified using classical morphologic methods. Finally, while most yeasts can be grown satisfactorily in or on a nutrient medium such as Sabouraud’s medium, molds can be more selective in their medium requirements. For example, a definitive diagnosis of Histoplasma capsulatum may require induction of the yeast or hyphal phase, which requires two different media and growth conditions, and can takes weeks to grow.
Yeast cells are typically identified biochemically in classical microbiology laboratories, often through commercial systems with a standardized substrate panel that is used for all yeast identifications. These systems may be automated (e.g., Vitek, bioMérieux, Inc., Durham, NC) or manual (e.g., API 20 C AUX, bioMérieux, Inc.). Cultures that grow as molds are typically identified based on morphological characteristics, and comprehensive mold identification skills usually require specific training in mycology. Presently, there are few programs that provide this type of education (15), which has resulted in only rudimentary identification skills typically learned on the job or through limited laboratory or workshop training. In fact, the lack of trained mycologists in clinical laboratories is an ongoing problem that continues to get worse (15) and, ironically, has further increased the urgency for the development of new fungal diagnostic assays that rely less on formal mycological training. An alternative solution to the lack of technicians with a background in mycology is the development of new assays that are panfungal, such that any potential pathogenic fungus’ profile is within the diagnostic capability (e.g., database) of the assay. Examples would be matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) or ribosomal sequencing, such as the internal transcribed spacer (ITS) region and/or a region of the large ribosomal subunit (D1/D2).
ASSAY SCOPE
One of the most challenging decisions in new assay development is which fungi should the assay be able to identify. Assays that only identified Candida spp. would capture the vast majority of human systemic fungal infections and reflect strategic preferences based on financial projections. Inclusion of common systemic fungal pathogens in an assay (Candida, Histoplasma, Cryptococcus, Pneumocystis, Fusarium, Coccidioides, Aspergillus) would seem to be of great value to a clinical laboratory. Depending on patient type or geographic region, some of these fungi might never, or rarely, be encountered, so inclusion of the other fungi could be wasteful or time-consuming, depending on the type of assay. Moreover, fungi that are rarely encountered can require the quickest identification due to their invasiveness or potentially high morbidity and mortality rates. For example, rhinocerebral mucormycosis, an acute mycotic infection of the sinuses, brain, and mouth, is caused by a number of mucoralean species and can be found in patients with poorly managed diabetes and other immunosuppressed patients. For these infections, surgery is often required, and a delay in diagnosis of even a few hours can be life-threatening. Because these infections are generally rare, developing specific assays for their identification can be financially risky for companies. For rhinocerebral mucormycosis, diagnosis would generally rely on patient history, known risk factors, symptoms, and pathology. Even then, a species level identification would be nearly impossible without a live culture. However, for other types of immunosuppressed patients, a differential diagnosis may be even more challenging, leading clinicians to rely on histopathology or live culture, which can be inconclusive, greatly delayed, or not possible.
Beyond a handful of major human fungal pathogens, few if any FDA-approved specific diagnostic tests exist beyond classical microbiological testing, and it is unlikely that a specific diagnostic assay would be cost-effective for fungi that are important but rarely seen. This lack of tests for a specific fungal infection argues for future development to investigate panfungal assays. These assays would almost certainly be sequence based in order to take advantage of the massive amount of sequence data already available. However, the ability to obtain pure culture allows non-nucleic acid-based assays, such as the macromolecule-based method, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), to be used. Because this assay interrogates a reference library with assay-generated spectral data, the library can be continuously expanded with new reference cultures as has been recently done for Candida auris (16). The advantage of a panfungal assay is that a prior index of suspicion about the causative agent arising from culture characteristics or patient history is not needed to select a specific assay due to the one-size-fits-all nature of these assays. However, an underlying major problem to getting a panfungal assay through the approval process would be validating the assay against all potential fungi that the assay might be tasked with identifying. Generally, culture collections are the only entities that might hold a substantial number of human pathogenic reference cultures. However, the cost of a single culture, depending on collection, is in the hundreds of dollars, making the validation step extremely expensive for just a single culture, let alone multiple isolates. In some cases, a fungus could be so rare that obtaining multiple cultures may not be possible.
FUNGAL TAXONOMY AND NOMENCLATURE
The recent change in the International Code for Botanical Nomenclature, now known as the International Code of Nomenclature for algae, fungi, and plants, leading to “one fungus one name,” removed the issue of having two names (anamorph and teleomorph) for some fungi (17). The previous naming system was extremely problematic for medically important fungi and the clinicians that have to keep up with identifying and treating infections caused by them. The explosion of molecular data and subsequent taxonomic analysis and reanalysis of phylogenetically informative sequences has led to even more debate regarding how to name and describe fungal pathogens. On the one hand, exquisite taxonomic detail derived from DNA sequencing has resulted in classifications below the species level (i.e., subspecies, varieties, etc.) or the designation of new species. This detail can be useful in research and sometimes, as in the case of differences in antifungal susceptibility or virulence, is imperative. For example, while treating an invasive aspergillosis infection, Balajee et al. discovered a morphologically indistinguishable sibling species of Aspergillus fumigatus, which was named Aspergillus lentulus, based on reduced susceptibilities to multiple antifungal drugs (18). On the other hand, recognizing pathogenic fungi that display small sequence variation and little or no morphologic or biochemical variation, which together would not alter the treatment strategy, creates a cumbersome problem for clinicians and clinical microbiologists. Assay developers can be caught in the middle because, in an effort to show how sensitive a new assay is, reliably discriminating fungi at the species level can be an asset. However, keeping current with ever-changing fungal taxonomy and applying these changes accurately in accordance with accepted rules can be a liability if done incorrectly or not in a timely manner.
An example of how complicated this issue can be is the identification of Fusarium. These fungi are extremely important pathogens of plants and are one of the most common mycotoxin producers. In fact, out of the top 100 economically most important plants, 81 can be infected by Fusarium spp. (19). As a human pathogen, fusaria are broadly resistant to available drugs, and they can disseminate in the immunosuppressed to cause invasive and life-threatening infections (20). However, more than 60% cannot be identified using morphological methods (20). Sequence-based identification methods that do not use targets in the ribosomal locus are typically more complicated than routine ITS-based sequence identification because a second or even third sequence target other than the ITS region may need to be included for proper identification. For the fusaria, ITS sequence data alone cannot be used to identify most species. The additional targets are less conserved than ribosomal targets and may require genus-specific primers. Unfortunately, selection of which additional targets and primers to use may be dependent on the ITS sequence. Sequencing multiple loci in a clinical laboratory is often not feasible, particularly if the choice of the next sequence is dependent on the identity of the first, which can add substantial delay to an identification. Multilocus phylogenetic analyses provided a robust framework for identifying Fusarium by grouping species that are virtually indistinguishable morphologically into species complexes (21). Of the twenty or so Fusarium species complexes, the vast majority containing human pathogens are nested within four complexes, which include the Fusarium solani species complex (FSSC), Fusarium oxysporum species complex (FOSC), Fusarium fujikuroi species complex (FFSC), and the Fusarium incarnatum-equiseti species complex (FIESC) (21, 22). Similar classification strategies have been proposed for the pathogenic Cryptococcus spp. (23) as well as the aspergilli (24), which groups species into sections. Expecting new assays to keep up with, and incorporate, complex taxonomic changes is a formidable challenge now and in the foreseeable future.
COLONIZER VERSUS INVADER DISCRIMINATION
A problem for diagnosing many human mycoses is that the causative agents are often part of the normal human flora. For these fungi, e.g., Candida spp., background levels characteristic of normal host stasis need to be distinguished from invasive infection. An assay’s ability to make this distinction likely requires, for clinical specimens that are tested directly, a quantitative component or a readout that is deemed positive in an amount that is known to significantly exceed normal levels or which is associated with an infection. For example, the Fungitell assay (Associates of Cape Cod, Inc., East Falmouth, MA) measures fungal beta-d-glucan (BDG) levels in serum. Glucans are part of the cell walls of many fungi but are not found in humans. A positive result from this assay is ≥80 pg/ml, whereas 60 to 79 pg/ml is indeterminate, and ≤60 pg/ml is negative. However, results of this assay may need to be interpreted with caution depending on patient type, underlying disease conditions, and fungus that is being detected. Mokaddas et al. used this assay in a study of pediatric cancer patients and found BDG levels higher than the positive threshold in some colonized patients (detected by swab) that did not develop candidemia and who were negative with other assays (25). PCR-based assays may have more challenges in distinguishing colonization from invasion because of the amplification nature of the assay and may be most valuable when employed for negative predictive value or in combination with other assays.
Fungi that are common in the environment also pose quantitative problems, with Aspergillus spp. being one of the most difficult to determine whether it is colonizing or invading, the so called “bystanders or pathogens” problem (26). For pulmonary aspergillosis, this distinction can be challenging as Aspergillus spp. spores are inhaled regularly, and the fungus can be cultured routinely from sputum, which itself can be contaminated with fungi in healthy patients. Because Aspergillus sp. do not circulate frequently in the blood, invasive diagnostic methods, such as biopsy, or less sensitive methods such as imaging, often must be used; however, some underlying diseases may preclude biopsy due to potential complications. In addition to the aspergilli, numerous other environmental fungi can be encountered normally in some clinical specimens. These may include members of Fusarium, Cryptococcus, Aureobasidium, Cladosporium, and Penicillium to name a few (27). However, in cases of trauma, where environmental fungi can be inoculated into tissue (28) but may not be invading, it is difficult to determine which fungus treatment should be directed against, as multiple species might be detected but only one may be invading tissue. Additionally, one of the most important advantages to having an assay with a quantitative component is the ability to determine whether antifungal treatment is working. This aspect is especially important when an infection is treated empirically since it allows an ineffective antifungal to be switched to an alternative.
COMBINING IDENTIFICATION AND ANTIFUNGAL SUSCEPTIBILITY TESTING IN A SINGLE ASSAY
Given the importance of time to diagnosis in infection, diagnostic microbiology is focusing increasingly on concurrent microbial identification and susceptibility testing. Separating these two assays adds an inordinate amount of time to infection management decision-making since identification would have to occur first, followed by determining the best antibiotic strategy. Resistance has been shown to occur in almost every major fungal pathogen, and it is a serious and still growing problem with Candida spp. In fact, both Candida albicans and Candida glabrata are on the World Health Organization antibiotic threat list (29). Some species, such as C. glabrata and C. auris can show innate resistance to some antifungals or can become resistant at fairly high frequencies. In the case of C. auris, resistance is problematic due to its high mortality rate, which is attributed in part to its tendency to appear in immunosuppressed patients who are already debilitated in their response to infection (30). Antifungal resistance in invasive aspergillosis is also becoming a major cause of concern, and it has been hypothesized to be due, in part, to the indiscriminate use of azoles in agriculture (31). In some regions, greater than 10% of aspergillosis is drug resistant (32). Some species of Aspergillus, such as Aspergillus terreus, have innate reduced susceptibility to some antifungals, making rapid identification paramount for good outcomes. Innate resistance is not uncommon in other fungi, such as Scedosporium or Fusarium; however, assays that combine both identification and susceptibility in a single test are limited and still early in development in fungi.
Presently, two general methods exist for susceptibility testing. These include phenotypic testing, which utilizes live culture to gauge growth in the presence of antibiotics, and genotypic testing, which looks for molecular markers associated with resistance. Diagnostic assays that combine the two are obviously complicated, although progress is being made for bacterial diagnosis. For examples, Accelerate Diagnostics, Inc. (Tucson, AZ) has a commercially available assay that utilizes a fluorescent in situ hybridization (FISH) component for identification and time lapse microscopy to measure susceptibility, all of which can be completed in ∼5 h (33). The assay works on a variety of bacteria (identification and susceptibility) as well as two species of Candida (identification only).
In contrast to phenotypic assays, genotypic assays do not require a growth component and likely could perform identification and susceptibility simultaneously. Whole-genome sequencing is a likely candidate for this role; however, it is probably years away, as template preparation, in the form of libraries to be sequenced, and bioinformatic analysis that could output identification and markers of antifungal resistance with little or no data manipulation would be challenging. Additionally, the presence of a specific biomarker, such as a single nucleotide polymorphism (SNP) associated with resistance, does not necessarily mean resistance would be expressed given that numerous biomarkers can be associated with drug resistance, individually or collectively. MALDI-TOF MS, conversely, may be more feasible, as it currently has been used to explore resistance and identification in C. albicans and C. glabrata (34). However, studies have focused mainly on comparing spectra produced when yeast cultures are grown in different levels of antifungal (35), which could be costly or time-consuming.
ADMINISTRATIVE CHALLENGES
Diagnosis of fungal infections relies heavily on the appropriate use of laboratory testing for effective treatment to be initiated in a timely manner but can be hindered by a number of factors, including lack of personnel with specific mycology training, lack of suitable diagnostic alternatives to live culture, and difficulties in standardizing these alternative diagnostic options. The need for timely diagnosis also aligns with the goals of an institution’s antimicrobial stewardship program (ASP), which is designed to optimize patient outcomes while minimizing antimicrobial harm, such as toxicity, unfavorable drug-drug interactions, and selection for resistant organisms (36). Antimicrobial stewardship programs and prescribing physicians depend on information and guidance from the clinical microbiology laboratory in order to accomplish these goals. While stewardship is critical for all infectious agents, fungi offer some unique problems. Treatment options are much more limited for fungi than for bacteria due to the limited antifungal repertoire, and the few remaining choices in cases of resistance, such as amphotericin B, can have severe side effects.
There are many challenges that clinical laboratories face when asked to bring in new laboratory testing for fungal identification. The clinical laboratory has witnessed a shift away from classic morphologic identification of microorganisms to more molecular and proteomic-based methods. However, PCR assays have not reached the same level of acceptance for the detection of human fungal pathogens as for other microorganisms, mainly because the low amount of fungal DNA in clinical specimens often challenges the detection limits of PCR. One of the most common questions facing laboratories when considering new fungal diagnostic tests is deciding which specific assay best suits the needs of the lab, its patients, and its providers. The increasing diversity of fungal pathogens poses a significant challenge in choosing the right test (37), which can be exacerbated by trying to keep current with the seemingly constant reorganization of fungal taxonomy. Because PCR assays are directed at a single or a few species, the clinician needs to have an index of suspicion with regard to a given species prior to selecting a specific PCR assay, which can make the assay confirmatory in nature.
Implementation of diagnostic platforms in the clinical microbiology laboratory requires careful consideration of personnel requirements, ease of use and cost of an instrument if one is required, facility requirements, workflow design, and institutional buy-in. The number and types of fungi that come into the laboratory for identification can affect this decision-making process. Importantly, fungi that come into the laboratory can be greatly affected by geography, as many are geographically restricted. Coccidioides spp., for example, might be frequently seen in the desert southwest of the United States but rarely if ever seen in the northeast, which would argue for keeping a specific diagnostic assay for this fungus on hand in an Arizona clinical laboratory but not in Maine. In fact, diagnosing endemic fungal infections in the United States, such as coccidioidomycosis and histoplasmosis, is a serious enough issue that the National Institutes of Health has recently released funding opportunities for diagnostic assays that specifically target this class of fungi. Alternatively, patient type can affect the spectrum of fungi seen in clinical specimens. Aspergillus and Fusarium might be expected in clinical laboratories that support cancer or transplant patients. Trauma centers, particularly if they support combat patients, might see elevated levels of Mucorales. In fact, these centers might consider sequence-based identification approaches, as well as the associated equipment and technician costs, as identification platforms due to the rarity of these fungi and the difficulty in identifying them to the species level. However, the commercial assays currently available for fungal identification are generally not comprehensive and typically only detect one or a few fungal pathogens at a time (38). MALDI-TOF MS is an exception, however. It requires an existing internal database that is interrogated by the mass spectra produced from unknown specimens. The sensitivities of these assays are also low, meaning that the fungal burden needs to be relatively high for the assay to be able to identify the fungus if an assay can even be used directly on clinical specimens. The limitations of the methodologies currently used for diagnosing invasive fungal infections often force clinicians to take an empirical approach to antifungal therapy, which has led to both an increase in antifungal resistance as well as time to diagnosis and illustrates the need for better assays to detect and identify fungal pathogens in a timely manner (39).
Finally, no assay can be implemented without considering cost savings. Cost savings at the bedside and in the laboratory are a win-win for all parties involved. Intuitively, if a diagnosis is provided earlier and is more accurate and reliable, patients who require antifungal therapy will receive it sooner, potentially shortening the length of their hospital stay, decreasing chances of acquiring nosocomial infections, and decreasing mortality rates (40). This outcome will lead to a cost savings for the patient and will have a positive financial impact on the institution. Most importantly, patient outcomes will be substantially improved with a faster and more accurate laboratory test for fungal identification.
CONCLUSIONS
Diagnosing fungal infections has always been challenging for numerous reasons, some of which overlap with bacterial diagnosis. However, the unique nature of fungi and the general rarity of infection compared to that with bacteria have resulted in major impediments to rapid, comprehensive assays for fungal identification. Some of the extreme challenges will continue to hold back the field of fungal diagnosis. Solving these challenges will require new advances in technology and a more creative application of current technologies to clinical problems. Conversely, better education and training as well as novel ways to introduce and utilize new tests in the microbiology laboratory may be one of the easier ways to improve fungal diagnosis.
ACKNOWLEDGMENTS
We thank David Kadosh for the Candida albicans images.
B.L.W. is supported by R21 AI128479-01A1 and R21 AI146700-01.
REFERENCES
- 1.Kozel TR, Wickes B. 2014. Fungal diagnostics. Cold Spring Harb Perspect Med 4:a019299. doi: 10.1101/cshperspect.a019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy J, Harris J, Gade L, Sehulster L, Newhouse E, O'Connell H, Noble-Wang J, Rao C, Balajee SA, Chiller T. 2014. Mucormycosis outbreak associated with hospital linens. Pediatr Infect Dis J 33:472–476. doi: 10.1097/INF.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 3.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, Etienne K, Deak E, Derado G, Shieh WJ, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367:2214–2225. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 4.Larone DH, Walsh TJ. 2013. Exserohilum rostratum: anatomy of a national outbreak of fungal meningitis. Clin Microbiol News 35:185–193. doi: 10.1016/j.clinmicnews.2013.11.001. [DOI] [Google Scholar]
- 5.Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, Candida auris Incident Management Team, Manuel R, Brown CS. 2018. Candida auris: a review of the literature. Clin Microbiol Rev 31:e00029-17. doi: 10.1128/CMR.00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparagano O, Foggett S. 2009. Diagnosis of clinically relevant fungi in medicine and veterinary sciences. Adv Appl Microbiol 66:29–52. doi: 10.1016/S0065-2164(08)00802-2. [DOI] [PubMed] [Google Scholar]
- 7.Arastehfar A, Wickes BL, Ilkit M, Pincus DH, Daneshnia F, Pan W, Fang W, Boekhout T. 2019. Identification of mycoses in developing countries. J Fungi (Basel) 5:E90. doi: 10.3390/jof5040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincus DH, Orenga S, Chatellier S. 2007. Yeast identification–past, present, and future methods. Med Mycol 45:97–121. doi: 10.1080/13693780601059936. [DOI] [PubMed] [Google Scholar]
- 9.Lau A, Chen S, Sleiman S, Sorrell T. 2009. Current status and future perspectives on molecular and serological methods in diagnostic mycology. Future Microbiol 4:1185–1222. doi: 10.2217/fmb.09.70. [DOI] [PubMed] [Google Scholar]
- 10.Arastehfar A, Fang W, Daneshnia F, Al-Hatmi AM, Liao W, Pan W, Khan Z, Ahmad S, Rosam K, Lackner M, Lass-Flörl C, Hagen F, Boekhout T. 2019. Novel multiplex real-time quantitative PCR detecting system approach for direct detection of Candida auris and its relatives in spiked serum samples. Future Microbiol 14:33–45. doi: 10.2217/fmb-2018-0227. [DOI] [PubMed] [Google Scholar]
- 11.Inglis PW, Pappas MCR, Resende LV, Grattapaglia D. 2018. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS One 13:e0206085. doi: 10.1371/journal.pone.0206085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czurda S, Smelik S, Preuner-Stix S, Nogueira F, Lion T. 2016. Occurrence of fungal DNA contamination in PCR reagents: approaches to control and decontamination. J Clin Microbiol 54:148–152. doi: 10.1128/JCM.02112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner K, Springer B, Pires VP, Keller PM. 2018. Molecular detection of fungal pathogens in clinical specimens by 18S rDNA high-throughput screening in comparison to ITS PCR and culture. Sci Rep 8:6964. doi: 10.1038/s41598-018-25129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry MD, White PL, Barnes RA. 2014. Comparison of four automated nucleic acid extraction platforms for the recovery of DNA from Aspergillus fumigatus. J Med Microbiol 63:1160–1166. doi: 10.1099/jmm.0.076315-0. [DOI] [PubMed] [Google Scholar]
- 15.Steinbach WJ, Mitchell TG, Schell WA, Espinel-Ingroff A, Coico RF, Walsh TJ, Perfect JR. 2003. Status of medical mycology education. Med Mycol 41:457–467. doi: 10.1080/13693780310001631322. [DOI] [PubMed] [Google Scholar]
- 16.Bao JR, Master RN, Azad KN, Schwab DA, Clark RB, Jones RS, Moore EC, Shier KL. 2018. Rapid, accurate identification of Candida auris by using a novel matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) database (library). J Clin Microbiol 56: e01700-17. doi: 10.1128/JCM.01700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawksworth DL. 2011. A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2:155–162. doi: 10.5598/imafungus.2011.02.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. 2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell 4:625–632. doi: 10.1128/EC.4.3.625-632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moretti AN. 2009. Taxonomy of Fusarium genus, a continuous fight between lumpers and splitters. Zb Mat Srp Prir Nauk 117:7–13. doi: 10.2298/ZMSPN0917007M. [DOI] [Google Scholar]
- 20.O'Donnell K, Sutton DA, Rinaldi MG, Sarver BAJ, Balajee SA, Schroers H-J, Summerbell RC, Robert VARG, Crous PW, Zhang N, Aoki T, Jung K, Park J, Lee Y-H, Kang S, Park B, Geiser DM. 2010. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol 48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Diepeningen A, Al-Hatmi AMS, Brankovics B, Sybren de Hoog G. 2014. Taxonomy and clinical spectra of Fusarium species: where do we stand in 2014? Curr Clin Micro Rep 1:10–18. doi: 10.1007/s40588-014-0003-x. [DOI] [Google Scholar]
- 22.O'Donnell K, Rooney AP, Proctor RH, Brown DW, McCormick SP, Ward TJ, Frandsen RJN, Lysøe E, Rehner SA, Aoki T, Robert VARG, Crous PW, Groenewald JZ, Kang S, Geiser DM. 2013. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet Biol 52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, Bicanic TA, Castaneda E, Chang YC, Chen J, Cogliati M, Dromer F, Ellis D, Filler SG, Fisher MC, Harrison TS, Holland SM, Kohno S, Kronstad JW, Lazera M, Levitz SM, Lionakis MS, May RC, Ngamskulrongroj P, Pappas PG, Perfect JR, Rickerts V, Sorrell TC, Walsh TJ, Williamson PR, Xu J, Zelazny AM, Casadevall A. 2017. The case for adopting the “species complex” nomenclature for the etiologic agents of cryptococcosis. mSphere 2:e00357-16. doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gams W, Christensen M, Onions AH. 1985. Infrageneric taxa of Aspergillus, p 55–62. In Samson RA, Pitt JI (ed), Advances in Penicillium and Aspergillus systematics. Plenum Press, New York, NY. [Google Scholar]
- 25.Mokaddas E, Burhamah MH, Khan ZU, Ahmad S. 2010. Levels of (1→3)-beta-D-glucan, Candida mannan and Candida DNA in serum samples of pediatric cancer patients colonized with Candida species. BMC Infect Dis 10:292. doi: 10.1186/1471-2334-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chotirmall SH, McElvaney NG. 2014. Fungi in the cystic fibrosis lung: bystanders or pathogens? Int J Biochem Cell Biol 52:161–173. doi: 10.1016/j.biocel.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. 2010. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesan A, Wells J, Shaikh F, Peterson P, Bradley W, Carson L, Petfield J, Klassen-Fischer M, Akers K, Downing K, Bialek R, Tribble D, Wickes BL. 2019. Molecular detection of filamentous fungi in formalin-fixed paraffin-embedded specimens in invasive fungal wound infections is feasible with high specificity. J Clin Microbiol 58:e01259-19. doi: 10.1128/JCM.01259-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. 2014. Antimicrobial resistance: global report on surveillance 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 30.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger S, El Chazli Y, Babu AF, Coste AT. 2017. Azole resistance in Aspergillus fumigatus: a consequence of antifungal use in agriculture? Front Microbiol 8:1024. doi: 10.3389/fmicb.2017.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M. 2016. Triazole resistance in Aspergillus spp.: a worldwide problem? J Fungi 2:21. doi: 10.3390/jof2030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnham JP, Wallace MA, Fuller BM, Shupe A, Burnham CD, Kollef MH. 2019. Clinical effect of expedited pathogen identification and susceptibility testing for Gram-negative bacteremia and candidemia by use of the Accelerate Pheno system. J Appl Lab Med 3:569–579. doi: 10.1373/jalm.2018.027201. [DOI] [PubMed] [Google Scholar]
- 34.Florio W, Tavanti A, Ghelardi E, Lupetti A. 2018. MALDI-TOF MS applications to the detection of antifungal resistance: state of the art and future perspectives. Front Microbiol 9:2577. doi: 10.3389/fmicb.2018.02577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.OPATHY Consortium, Gabaldon T. 2019. Recent trends in molecular diagnostics of yeast infections: from PCR to NGS. FEMS Microbiol Rev 43:517–547. doi: 10.1093/femsre/fuz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alanio A, Bretagne S. 2014. Difficulties with molecular diagnostic tests for mould and yeast infections: where do we stand? Clin Microbiol Infect 20(Suppl):36–41. doi: 10.1111/1469-0691.12617. [DOI] [PubMed] [Google Scholar]
- 37.Klutts JS, Robinson-Dunn B. 2011. A critical appraisal of the role of the clinical microbiology laboratory in diagnosis of invasive fungal infections. J Clin Microbiol 49:S39–S42. doi: 10.1128/JCM.00468-11. [DOI] [Google Scholar]
- 38.Wolk DW, Dunne WM. 2011. New technologies in clinical microbiology. J Clin Microbiol 49:S62–S67. doi: 10.1128/JCM.00834-11. [DOI] [Google Scholar]
- 39.Enoch DA, Ludlam HA, Brown NM. 2006. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol 55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- 40.Fournier PE, Drancourt M, Colson P, Rolain JM, La Scola B, Raoult D. 2013. Modern clinical microbiology: new challenges and solutions. Nat Rev Microbiol 11:574–585. doi: 10.1038/nrmicro3068. [DOI] [PMC free article] [PubMed] [Google Scholar]