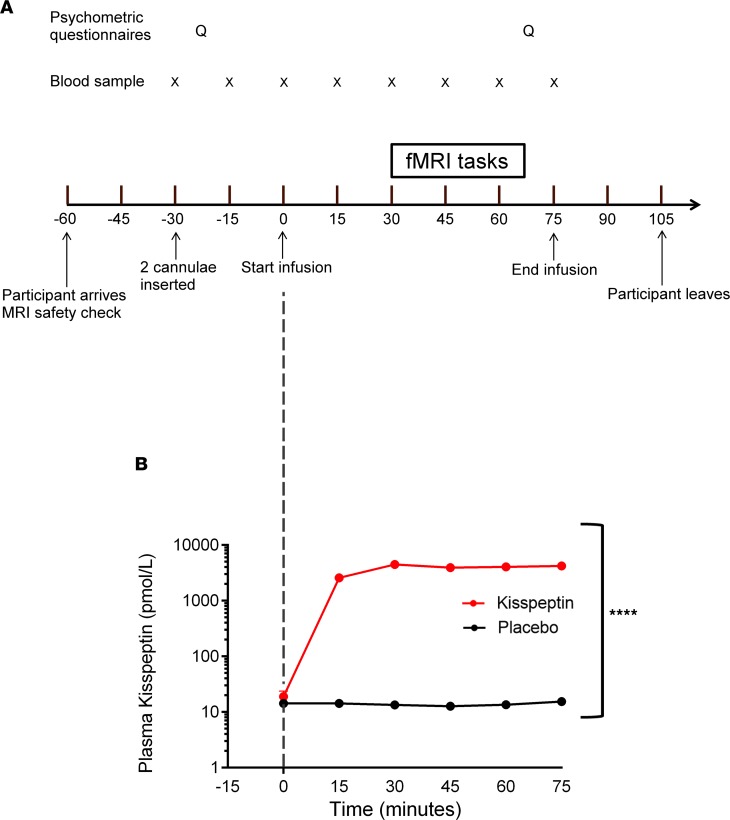
Figure 1. Experimental protocol and effects of kisspeptin administration on circulating kisspeptin levels.
(A) Thirty-three healthy young men participated in a randomized, double-blind, 2-way crossover, placebo-controlled study. They attended 2 study visits: 1 for intravenous administration of kisspeptin (1 nmol/kg/h) and 1 for intravenous administration of an equivalent volume of placebo (vehicle) for 75 minutes. Blood samples were taken every 15 minutes (X). Participants completed baseline and intrainfusion psychometric questionnaires (Q) and underwent functional MRI (fMRI) scanning while performing olfactory and facial attractiveness tasks. (B) Kisspeptin infusion resulted in increased circulating kisspeptin levels (****P < 0.0001, and n = 33), reaching a plateau at 30 minutes after initiation, with stable circulating kisspeptin levels during the fMRI and intrainfusion psychometric questionnaires.