Abstract
PURPOSE
The Congress of Neurological Surgeons (CNS) has developed a series of guidelines for the treatment of adults with metastatic brain tumors, including systemic therapy and supportive care topics. ASCO has a policy and set of procedures for endorsing clinical practice guidelines that have been developed by other professional organizations.
METHODS
Two CNS guidelines were reviewed for developmental rigor by methodologists, and an independent multidisciplinary Expert Panel was formed to review the content and assess agreement with the recommendations. The Expert Panel voted to endorse the two guidelines, and ASCO and Society for Neuro-Oncology (SNO) independently reviewed and approved the ASCO/SNO guideline endorsement.
RESULTS
The ASCO/SNO Expert Panel determined that the recommendations from the CNS anticonvulsants and steroids guidelines, published January 9, 2019, are clear, thorough, and based on the most relevant scientific evidence. ASCO/SNO endorsed these two CNS guidelines with minor alterations.
RECOMMENDATIONS
Key recommendations include the following: prophylactic antiepileptic drugs were not recommended for routine use; and corticosteroids, specifically dexamethasone, were recommended for temporary symptomatic relief in patients with neurologic symptoms and signs related to mass effect from brain metastases. Additional information is available at www.asco.org/neurooncology-guidelines.
INTRODUCTION
The Congress of Neurological Surgeons (CNS) has recently published a series of guidelines for the treatment and care of adults with metastatic brain tumors.1 Before publication, CNS requested that ASCO provide feedback on and consider endorsing the guideline series. As the care of the target population of these guidelines is an important issue for the members of both ASCO and the Society for Neuro-Oncology (SNO), ASCO and SNO conducted a joint guideline endorsement process for these guidelines.
OVERVIEW OF THE ASCO GUIDELINE ENDORSEMENT PROCESS
ASCO has policies and procedures for endorsing practice guidelines that have been developed by other professional organizations. The goal of guideline endorsement is to increase the number of high-quality, ASCO-vetted guidelines available to the ASCO membership. The ASCO endorsement process involves an assessment by ASCO staff of candidate guidelines for methodologic quality using the Rigor of Development subscale of the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument. (See Methodology Supplement for more detail.)
Disclaimer
The clinical practice guidelines and other guidance published herein are provided by the American Society of Clinical Oncology, Inc. (“ASCO”) to assist providers in clinical decision making. The information therein should not be relied upon as being complete or accurate, nor should it be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. With the rapid development of scientific knowledge, new evidence may emerge between the time information is developed and when it is published or read. The information is not continually updated and may not reflect the most recent evidence. The information addresses only the topics specifically identified therein and is not applicable to other interventions, diseases, or stages of diseases. This information does not mandate any particular course of medical care. Further, the information is not intended to substitute for the independent professional judgment of the treating provider, as the information does not account for individual variation among patients. Recommendations reflect high, moderate, or low confidence that the recommendation reflects the net effect of a given course of action. The use of words like “must,” “must not,” “should,” and “should not” indicate that a course of action is recommended or not recommended for either most or many patients, but there is latitude for the treating physician to select other courses of action in individual cases. In all cases, the selected course of action should be considered by the treating provider in the context of treating the individual patient. Use of the information is voluntary. ASCO provides this information on an “as is” basis, and makes no warranty, express or implied, regarding the information. ASCO specifically disclaims any warranties of merchantability or fitness for a particular use or purpose. ASCO assumes no responsibility for any injury or damage to persons or property arising out of or related to any use of this information or for any errors or omissions.
THE BOTTOM LINE
Anticonvulsant Prophylaxis and Steroid Use in Adults with Metastatic Brain Tumors: ASCO and SNO Endorsement of the Congress of Neurological Surgeons Guidelines
ASCO and Society for Neuro-Oncology (SNO) endorse the Congress of Neurological Surgeons (CNS) Clinical Practice Guidelines, The Role of Prophylactic Anticonvulsants in the Treatment of Adults with Metastatic Brain Tumors and The Role of Steroids in the Treatment of Adults with Metastatic Brain Tumors, with some minor alterations.
Guideline Questions
CNS Anticonvulsant Guideline
Do prophylactic antiepileptic drugs decrease the risk of seizures in nonsurgical patients with brain metastases who are otherwise seizure free? Do prophylactic antiepileptic drugs decrease the risk of seizures in patients with brain metastases and no prior history of seizures in the postoperative setting?
CNS Steroids Guideline
Do steroids improve neurologic symptoms and/or quality of life in patients with metastatic brain tumors compared with supportive care only or other treatment options? If steroids are administered, what dose should be given?
Target Population
Adults with metastatic brain tumors.
Target Audience
Medical oncologists, neurologists, and others who provide care for adults with metastatic brain tumors.
Methods
An ASCO/SNO Expert Panel was convened to consider endorsing the CNS guideline recommendations that were based on a systematic review of the medical literature. The ASCO/SNO Expert Panel considered the methodology used in the CNS guidelines by considering the results from the Appraisal of Guidelines for Research and Evaluation II review instrument. The ASCO/SNO Expert Panel carefully reviewed the CNS guidelines content to determine its appropriateness for ASCO endorsement.
Recommendations
(Additions by the ASCO/SNO Expert Panel are in bold italics. See the note below regarding CNS recommendation levels.)
CNS Anticonvulsants Guideline2
Level 3: Prophylactic antiepileptic drugs are not recommended for routine use in patients with brain metastases who did not undergo surgical resection and who are otherwise seizure free.
Level 3: Routine postcraniotomy antiepileptic drug use for seizure-free patients with brain metastases is not recommended.
CNS Steroids Guideline5: Steroid Therapy Versus No Steroid Therapy
Patients with asymptomatic brain metastases without mass effect:
Insufficient evidence exists to make a treatment recommendation for this clinical scenario.
Patients with brain metastases with mild symptoms related to mass effect:
Level 3: Corticosteroids are recommended to provide temporary symptomatic relief of symptoms related to increased intracranial pressure and edema secondary to brain metastases. It is recommended for patients who are symptomatic from metastatic disease to the brain that a starting dose of dexamethasone 4 to 8 mg/d be considered.
Patients with brain metastases with moderate to severe symptoms related to mass effect:
Level 3: Corticosteroids are recommended to provide temporary symptomatic relief of symptoms related to increased intracranial pressure and edema secondary to brain metastases. If patients exhibit severe symptoms that are consistent with increased intracranial pressure, it is recommended that higher doses, such as 16 mg/d or more, be considered.
Choice of steroid:
Level 3: If corticosteroids are administered, dexamethasone is the best drug choice given the available evidence.
Duration of corticosteroid administration:
Level 3: Corticosteroids, if administered, should be tapered as rapidly as possible, but no faster than clinically tolerated, on the basis of an individualized treatment regimen and a full understanding of the long-term sequelae of corticosteroid therapy.
ASCO/SNO Expert Panel comment: The Panel’s expert opinion is that, given the important adverse effects of steroids, the minimum effective dose (often no more than 4 mg) should be used where possible and night-time doses of steroids should be avoided to minimize toxicity.
Note regarding CNS Level 3 recommendation classification: CNS defines a Level 3 recommendation as one based on “Evidence from case series, comparative studies with historical controls, case reports, and expert opinion, as well as significantly flawed randomized controlled trials.”
Additional Resources
More information, including a Data Supplement, a Methodology Supplement, slide sets, and clinical tools and resources, is available at www.asco.org/neurooncology-guidelines. Patient information is available at www.cancer.net
The CNS Guideline series can be found at https://www.cns.org/guidelines/guidelines-treatment-adults-metastatic-brain-tumors
ASCO believes that cancer clinical trials are vital to inform medical decisions and improve cancer care, and that all patients should have the opportunity to participate.
Guideline and Conflicts of Interest
A multidisciplinary Expert Panel was assembled in accordance with ASCO’s Conflict of Interest Policy Implementation for Clinical Practice Guidelines (“Policy,” found at http://www.asco.org/rwc). All members of the Expert Panel completed ASCO’s disclosure form, which requires disclosure of financial and other interests, including relationships with commercial entities that are reasonably likely to experience direct regulatory or commercial impact as a result of promulgation of the guideline. Categories for disclosure include employment; leadership; stock or other ownership; honoraria, consulting or advisory role; speaker's bureau; research funding; patents, royalties, other intellectual property; expert testimony; travel, accommodations, expenses; and other relationships. In accordance with the Policy, the majority of the members of the Expert Panel did not disclose any relationships constituting a conflict under the Policy.
CLINICAL QUESTION(S) AND TARGET POPULATION
CNS has published a series of eight guidelines1-9 that cover multiple aspects of the treatment of adults with metastatic brain tumors. Of these eight guidelines, four addressed systemic therapy and supportive care for these patients. Of the four guidelines that cover systemic therapy and supportive care, two were selected for endorsement—The Role of Prophylactic Anticonvulsants in the Treatment of Adults with Metastatic Brain Tumors2 and The Role of Steroids in the Treatment of Adults with Metastatic Brain Tumors.5 This work focuses on these two guidelines selected for endorsement. The two guidelines that were not endorsed reviewed the role of chemotherapy3 and various emerging therapies.4 Details on the newly identified evidence found and the review of the unendorsed guidelines can be found in Data Supplement 7. Because these two guidelines cover the same target population (adults with metastatic brain tumors) and because their recommendations have substantial interaction between the guidelines, ASCO conducted a common review of both guidelines. The complete set of clinical questions addressed by these two guidelines is provided in Supplemental Table 1. In summary, they address the role of anticonvulsant agents and steroids in the treatment of adults with brain metastases.
SUMMARY OF THE CNS GUIDELINE SERIES DEVELOPMENT METHODOLOGY
The CNS guideline series was developed by a multidisciplinary author expert panel, the Metastatic Brain Tumor Guidelines Task Force, which included medical oncologists, radiation oncologists, neurologic surgeons, neuro-oncologists, and others. It built on a previous guideline series published in 2010. The literature search of MEDLINE, Embase, the Cochrane Database of Systemic Reviews, and the Cochrane Central Registry of Controlled Trials ended in December 2015 and began either in 2008 or 1990 depending on whether the specific guideline was an update of a 2010 guideline or new. Details of search strategies and study inclusion criteria and outcomes of interest are available in the guideline publications.1,2,5
The evidence review conducted by CNS identified many studies for inclusion in the guideline’s qualitative synthesis of the literature, summarized in Data Supplement 4. Risk of bias and overall quality of the evidence, as well as the strength of the recommendations, were determined using CNS’s published methods (https://www.cns.org/guidelines/guideline-procedures-policies/guideline-development-methodology).
RESULTS OF THE ASCO/SNO INITIAL METHODOLOGY AND CONTENT EVALUATION
An initial methodology evaluation of the CNS guideline series was completed independently for each guideline in the series by two ASCO guideline staff members using the Rigor of Development subscale from the AGREE II instrument. Details of the scores are available in Data Supplement 6. Each guideline in the series was also initially assessed by two content evaluators, members of the SNO guideline committee, who conducted a structured evaluation of the clinical content of the guideline series. Based on AGREE II and the clinical evaluations, the ASCO Clinical Practice Guideline Committee determined that the guideline series as a whole warranted detailed review by an ASCO/SNO Expert Panel (Appendix Table A1, online only) to determine which guidelines/recommendations could be endorsed.
This is the most recent information as of the publication date. For updates, the most recent information, and to submit new evidence, please visit www.asco.org/neurooncology-guidelines.
METHODS AND RESULTS OF THE ASCO UPDATED LITERATURE REVIEW
This systematic review–based guideline product was developed by a multidisciplinary Expert Panel that included a patient representative and an ASCO guidelines staff member with health research methodology expertise. PubMed was searched from December 2015 to March 20, 2018. In addition, abstracts of the ASCO and American Society for Radiation Oncology annual meetings from 2016 to 2017 were searched for relevant randomized controlled trials. The updated search was guided by the signals10 approach, which was designed to identify only new, potentially practice-changing data (signals) that might translate into revised practice recommendations. The approach relies on targeted routine literature searching and the expertise of ASCO/SNO Expert Panel members to help identify potential signals. As there would likely be some overlap among included articles, the search was structured to update the entire CNS guideline series literature search at the same time, with identified articles then assigned to the relevant guideline. The search was restricted to articles that were published in English. In addition, it was further restricted (compared with the original CNS search) to prospective studies of more than 30 participants and retrospective studies of more than 100 participants. The CNS guideline series used lower cutoffs which varied from guideline to guideline; however, as a result of concerns raised by the Expert Panel co-chairs that relevant studies may have been missed in the CNS guideline searches, an additional search was conducted back to January 2000 looking for studies with only “CNS,” “central nervous system,” or similar terms, but not the keyword “brain.” The full search strategy and additional details can be found in Data Supplement 2. All funding for the administration of the project was provided by ASCO. The Methodology Supplement (available at www.asco.org/neurooncology-guidelines) provides additional information about the signals approach.
The updated search yielded 31 new articles. These articles are summarized in Data Supplement 1, Table 1. A review of these results found no new studies that were relevant to the anticonvulsants and steroids guidelines.
RESULTS OF THE ASCO/SNO CONTENT REVIEW
The ASCO/SNO Expert Panel reviewed the recommendations of the CNS guidelines in detail. Details of this review can be found in Data Supplement 4 and are summarized in this section.
Anticonvulsants
The Expert Panel found the recommendations reasonable, given the evidence, and hoped that although the guideline presents only negative recommendations it would still be useful in reducing the incidence of patients placed unnecessarily on anticonvulsant medications. The Expert Panel did decide to make a minor alteration to the first recommendation, adding the phrase, “for routine use,” to make it consistent with the second recommendation. This guideline was endorsed.
Steroids
The Expert Panel agreed with the recommendations in this guideline. This guideline was endorsed. However, the Expert Panel felt that the recommendations in the guideline did not fully address the important adverse effects of steroid use, which can greatly affect a patient’s quality of life, and added an additional comment encouraging the use of the minimal effective dose and avoiding night-time dosing. The Expert Panel also notes that a recent article by Arbor et al,11 published after the search conducted for this endorsement, suggests that higher doses of steroids may have negative consequences on outcomes in patients receiving programmed death ligand 1 inhibitors. This emerging literature should be monitored as it may affect the recommendations in future.
ENDORSEMENT RECOMMENDATION
ASCO and SNO endorse the CNS guidelines on anticonvulsants2 and steroids5 in the treatment of adults with brain metastases, with a minor alteration, as presented in the Bottom Line Box.
Related ASCO Guidelines
Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update (http://ascopubs.org/doi/10.1200/JCO.2018.79.2713)
ACKNOWLEDGMENT
The Expert Panel thanks Raetasha Dabney, MD, David Ollila, MD, and the Clinical Practice Guidelines Committee for thoughtful reviews and insightful comments on this guideline endorsement.
Appendix
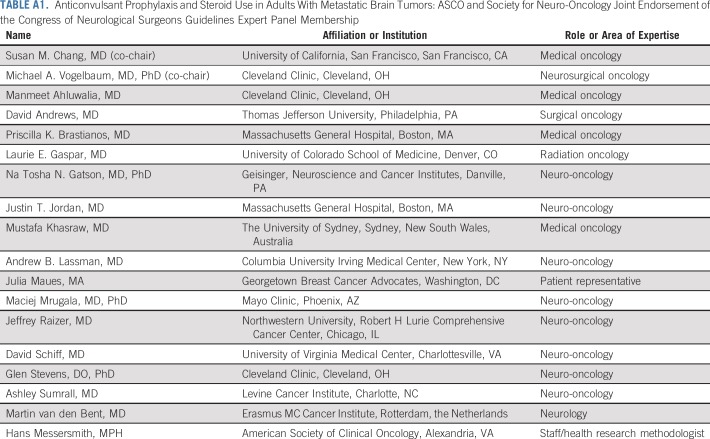
TABLE A1.
Anticonvulsant Prophylaxis and Steroid Use in Adults With Metastatic Brain Tumors: ASCO and Society for Neuro-Oncology Joint Endorsement of the Congress of Neurological Surgeons Guidelines Expert Panel Membership
Footnotes
Editor’s note:
This American Society of Clinical Oncology Clinical Practice Guideline provides recommendations, with comprehensive review and analyses of the relevant literature for each recommendation. Additional information, including a Data Supplement with additional evidence tables, a Methodology Supplement, slide sets, clinical tools and resources, and links to patient information at www.cancer.net, is available at www.asco.org/neurooncology-guidelines.
Clinical Practice Guidelines Committee approval: September 6, 2018
Reprint requests: 2318 Mill Road, Suite 800, Alexandria, VA 22314; guidelines@asco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Susan M. Chang, Manmeet Ahluwalia, Mustafa Khasraw, Jeffrey Raizer, David Schiff, Glen Stevens, Martin van den Bent, Michael A. Vogelbaum
Administrative support: Susan M. Chang, Priscilla K. Brastianos
Provision of study material or patients: Susan M. Chang
Collection and assembly of data: Manmeet Ahluwalia, Mustafa Khasraw, Maciej Mrugala, Jeffrey Raizer, David Schiff, Ashley Sumrall, Martin van den Bent
Data analysis and interpretation: Susan M. Chang, Hans Messersmith, Manmeet Ahluwalia, David Andrews, Priscilla K. Brastianos, Laurie E. Gaspar, Na Tosha N. Gatson, Justin T. Jordan, Mustafa Khasraw, Andrew B. Lassman, Julia Maues, Maciej Mrugala, Jeffrey Raizer, David Schiff, Glen Stevens, Martin van den Bent, Michael A. Vogelbaum
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Anticonvulsant Prophylaxis and Steroid Use in Adults With Metastatic Brain Tumors: ASCO and SNO Endorsement of the Congress of Neurological Surgeons Guidelines
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Susan M. Chang
Consulting or Advisory Role: Tocagen
Research Funding: Novartis (Inst), Agios (Inst)
Manmeet Ahluwalia
Stock and Other Ownership Interests: MimiVax
Honoraria: Prime Oncology, Elsevier, Itamar Medical (I)
Consulting or Advisory Role: Monteris Medical, AstraZeneca, Bristol-Myers Squibb, AbbVie, CBT Pharmaceuticals, Kadmon, VBI Vaccines
Research Funding: Novartis, TRACON Pharma, Novocure, Spectrum Pharmaceuticals, Eli Lilly, ImClone, Boehringer Ingelheim, AstraZeneca
David Andrews
Employment: Imvax
Leadership: Imvax
Stock and Other Ownership Interests: Imvax
Consulting or Advisory Role: Imvax
Patents, Royalties, Other Intellectual Property: Thomas Jefferson University holds the patents and Imvax has the exclusive rights to the license
Priscilla K. Brastianos
Honoraria: Merck, Genentech, Angiochem
Consulting or Advisory Role: Genentech, Eli Lilly
Advisory Role: Angiochem
Research Funding: Merck, Pfizer
Laurie E. Gaspar
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Other Relationship: American Association of Neurosurgery, National Cancer Institute Thoracic Malignancy Steering Committee
Justin T. Jordan
Patents, Royalties, Other Intellectual Property: Textbook royalties
Other Relationship: Shepherd Therapeutics, Shepherd Foundation, NF Network
Mustafa Khasraw
Honoraria: Pfizer, Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, AbbVie, Eli Lilly
Research Funding: AbbVie (Inst), Bristol-Myers Squibb (Inst), Specialised Therapeutics (Inst)
Travel, Accommodations, Expenses: Genentech
Andrew B. Lassman
Honoraria: Prime Oncology, WebMD, American Society of Clinical Oncology, Italian Association for Cancer Research, Focus Forward Incentives, Research America, Gerson Lehrman Group, Guidepoint Global, Health Advisors Bureau, Medefield, MedSurvey, Olson Research Group, SAI MedPartners, Schlesinger Associates, Market Strategies International, Defined Health, Marketplusresearch.com, AbbVie
Consulting or Advisory Role: BioClinica, AbbVie, Sapience Therapeutics, AstraZeneca, Novocure, Kadmon, Cortice, Celgene, Agios
Research Funding: AbbVie (Inst), Novartis (Inst), Karyopharm Therapeutics (Inst), Genentech (Inst), Novocure (Inst), Aeterna Zentaris (Inst), Pfizer (Inst), Bayer (Inst), Onyx Pharmaceuticals (Inst), Agenus (Inst), GlaxoSmithKline (Inst), Stemline Therapeutics (Inst), Northwest Biotherapeutics (Inst), Plexxikon (Inst), Tocagen (Inst), Regeneron (Inst), VBL Therapeutics (Inst), e-Therapeutics (Inst), Bristol-Myers Squibb (Inst), ImmunoCellular Therapeutics (Inst), Merck (Inst), Amgen (Inst), Celldex (Inst), Millennium Pharmaceuticals (Inst), MedImmune (Inst), Boehringer Ingelheim (Inst), Kadmon (Inst), RTOG Foundation (Inst), Boston Biomedical (Inst), BeiGene (Inst), Diffusion Pharmaceuticals (Inst), Agios (Inst), Celgene (Inst), Vascular Biogenics (Inst), Angiochem (Inst), Northwest Biotherapeutics (Inst), Orbus (Inst), VBI Vaccines (Inst)
Travel, Accommodations, Expenses: Karyopharm Therapeutics, Tocagen, Radiological Society of North America, AbbVie, Agios, Novocure, NRG Oncology Foundation, Celgene, Kadmon, Bristol-Myers Squibb
Other Relationship: Medical expert in malpractice/disability cases
Maciej Mrugala
Consulting or Advisory Role: Novocure, AbbVie
Research Funding: Arbor Pharmaceuticals
Travel, Accommodations, Expenses: AbbVie, Novocure
Jeffrey Raizer
Employment: Agenus, Celldex, Astellas Pharma
Stock and Other Ownership Interests: Agenus, Exicure, Cancer Action Now, Celldex
Honoraria: ZIOPHARM Oncology
Consulting or Advisory Role: ZIOPHARM Oncology
Research Funding: Novartis, Genentech, Plexxikon, Merck, Stemline Therapeutics, AstraZeneca, MedImmune
David Schiff
Consulting or Advisory Role: Orbus Therapeutics, Monteris Medical, Cavion (Inst)
Research Funding: Bayer (Inst)
Ashley Sumrall
Stock and Other Ownership Interests: Novocure (I)
Honoraria: Gerson Lehrman Group, Cardinal Health
Consulting or Advisory Role: Novocure, Amgen, AbbVie, Cardinal Health, Merck
Speakers' Bureau: Bristol-Myers Squibb, Novocure, Prime Oncology
Research Funding: Bristol-Myers Squibb (Inst), Novocure (Inst)
Travel, Accommodations, Expenses: Novocure, Bristol-Myers Squibb, Xcenda, American Association of Neurologic Surgeons
Martin van den Bent
Consulting or Advisory Role: AbbVie, Celldex, Bristol-Myers Squibb, MSD, Daiichi Sankyo, Celgene, Agios
Research Funding: AbbVie (Inst)
Michael A. Vogelbaum
Stock and Other Ownership Interests: Infusion Therapeutics
Honoraria: Medicenna
Research Funding: Tocagen (Inst), DNAtrix (Inst), Medicenna (Inst), Infusion Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Patents for drug delivery devices for the brain
Travel, Accommodations, Expenses: Infusion Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Olson JJ, Kalkanis SN, Ryken TC. Congress of Neurological Surgeons systematic review and evidence-based guidelines for the treatment of adults with metastatic brain tumors: Executive summary. Neurosurgery. doi: 10.1093/neuros/nyy540. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Rennert RC, Olson JJ. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of prophylactic anticonvulsants in the treatment of adults with metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy545. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 3.Sherman JH, Simon SL, Harrod T, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of chemotherapy in the management of adults with newly diagnosed metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy544. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Elder JB, Nahed BV, Linskey ME, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of emerging and investigational therapies for the treatment of adults with metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy547. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Ryken TC, Kuo JS, Prabhu RS, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of steroids in the treatment of adults with metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy546. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Ammirati MA, Nahed BV, Andrews D, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on treatment options for adults with multiple brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy548. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Graber JJ, Cobbs CS, Olson JJ. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the use of stereotactic radiosurgery in the treatment of adults with metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy543. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Nahed BV, Alvarez-Breckenridge C, Brastianos P, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of surgery in the management of adults with metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy542. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar LE, Prabhu RS, Hdeib A, et al. Congress of Neurological Surgeons systematic review and evidence-based guidelines on the role of whole brain radiation therapy in adults with newly diagnosed metastatic brain tumors. Neurosurgery. doi: 10.1093/neuros/nyy541. epub ahead of print on January 9, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Shojania KG, Sampson M, Ansari MT, et al. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007;147:224–233. doi: 10.7326/0003-4819-147-4-200708210-00179. [DOI] [PubMed] [Google Scholar]
- 11.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non–small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]