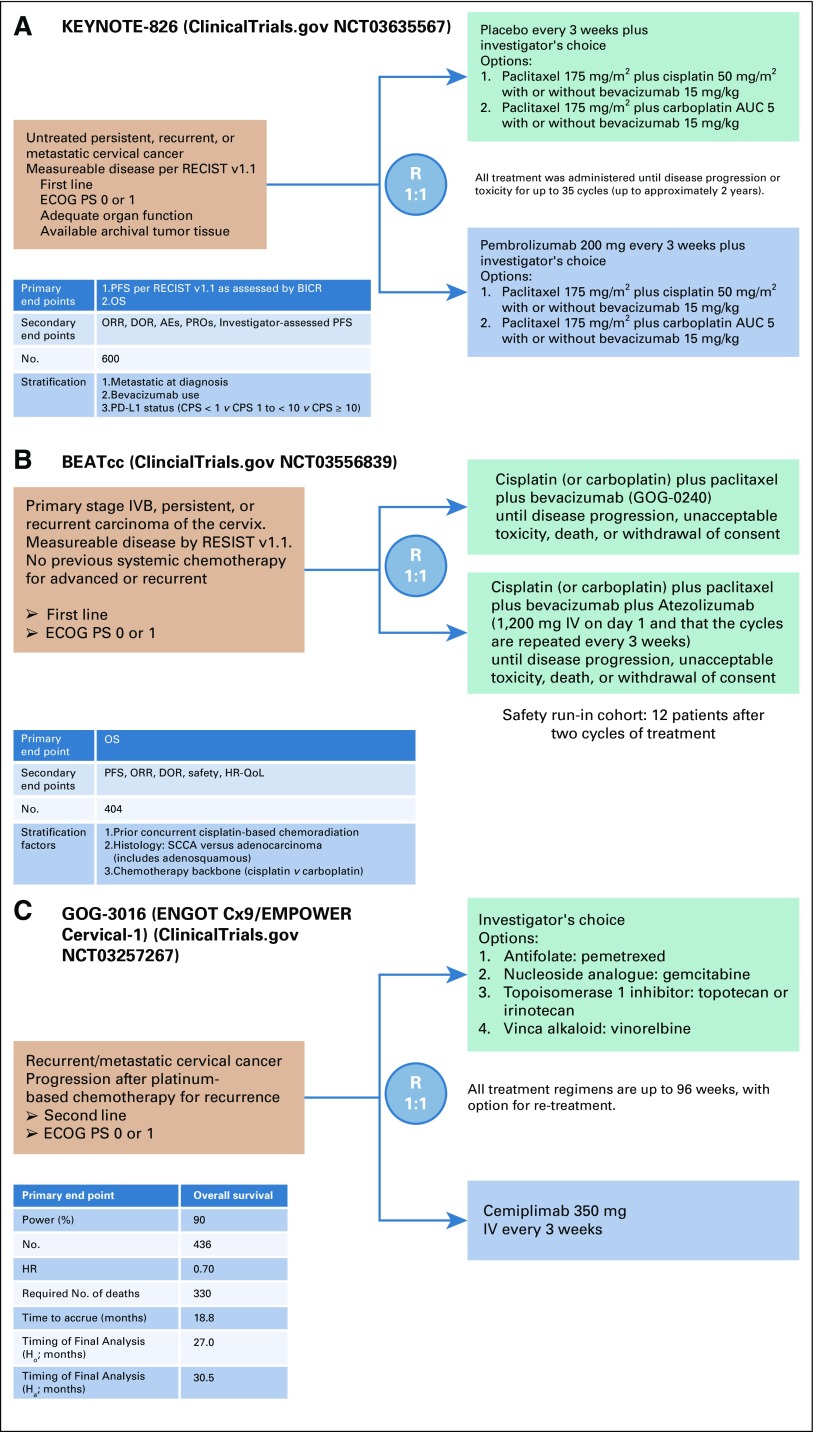
FIG 3.
Ongoing phase III, randomized clinical trials for women with recurrent/metastatic cervical cancer. (A) KEYNOTE-826 (Keytruda Trial 826) trial schema of first-line chemotherapy with or without bevacizumab versus chemotherapy with or without bevacizumab plus pembrolizumab. (B) BEATcc (Beat Cervical Cancer trial) trial schema of first-line chemotherapy plus bevacizumab with and without atezolizumab. (C) GOG-0316 (Gynecologic Oncology Group study 3016) trial schema of cemiplimab versus physician’s choice chemotherapy after progression receiving primary platinum-based therapy. AE, adverse events; AUC, area under the concentration-time curve; BICR, blinded independent central review; CPS, combined positive score; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; IV, intravenously; ORR, overall response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PROs, patient-reported outcomes; QoL, quality of life; R, randomize; RECIST v 1.1, Response Evaluation Criteria in Solid Tumors version 1.1; SCCA, squamous cell carcinoma.