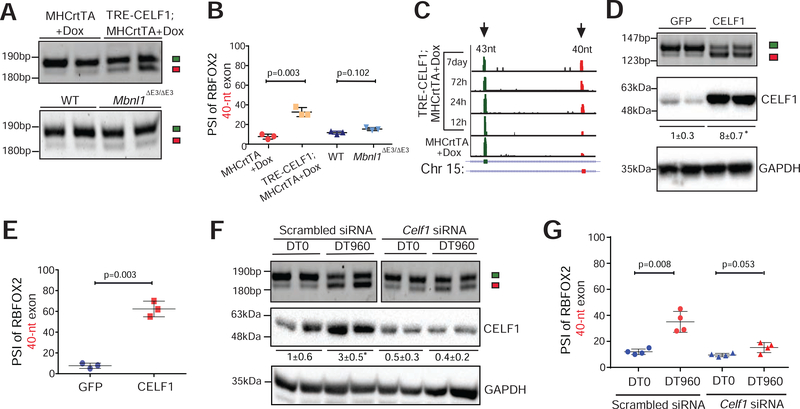
Figure 3. CELF1 upregulation promotes the production of non-muscle RBFOX240 isoform in the heart.
(A) RT-PCR analysis of Rbfox2 43-nt and 40-nt exons in hearts of tet-inducible, heart-specific CELF1 bitransgenics (TRE-CELF1; MHCrtTA) and littermate control mice induced with 6g/kg Dox for ten days, as well as in hearts of wildtype (WT) and Mbnl1ΔE3/ΔE3 mice. (B) PSI values of Rbfox2 40-nt exon from the indicated genotypes in A. n=4 mice for each genotype. (C) RNA-seq read density across Rbfox2 43-nt and 40-nt exons from mouse hearts following CELF1 overexpression at indicated time points (Wang et al., 2015). (D) RT-PCR analysis of Rbfox2 43-nt and 40-nt exons (top), and immunoblot analysis of CELF1 protein normalized to GAPDH (bottom) in HL-1 cells following infection with GFP or CELF1 expressing adenoviruses. (E) PSI values of Rbfox2 40-nt exon from D. n=4 independent infections. (F) RT-PCR analysis of Rbfox2 43-nt and 40-nt exons (top) and immunoblot analysis of CELF1 protein normalized to GAPDH (bottom) in HL-1 cells after co-transfection with scrambled control or Celf1 targeting siRNA(s), and DT0 or DT960 plasmids. (G) PSI values of Rbfox2 40-nt exon from F. n=4 independent transfections. All data are mean ± s.d., and p-values were derived from a parametric t-test (two-sided, unpaired), with Welch’s correction.