Abstract
Medulloblastoma (MB) is the most common malignant childhood tumor of the brain. Multimodal treatment consisting of surgery, radiation therapy, and chemotherapy reduced cumulative incidence of late mortality but increased the incidence of subsequent neoplasms and severe, incapacitating chronic health conditions. Present treatment strategies fail to recognize heterogeneity within patients despite wide divergence in individual responses. The persistent mortality rates and serious side effects of non-targeted cytotoxic therapies indicate a need for more refined therapeutic approaches. Advanced genomic research has led to the accumulation of an enormous amount of genetic information and resulted in a consensus distinguishing four molecular subgroups, WNT-activated, SHH-activated, and Group 3 and 4 medulloblastomas. These have distinct origin, demographics, molecular alterations, and clinical outcomes. Although subgroup affiliation does not predict response to therapy, new subgroup-specific markers of prognosis can enable a more layered risk stratification with additional subtypes within each primary subgroup. Here, we summarize subgroup-specific genetic alterations and their utility in current treatment strategies. The transition toward molecularly targeted interventions for newly diagnosed MBs remains slow, and prospective trials are needed to confirm stratifications based on molecular alterations. At the same time, numerous studies focus at fine-tuning the intensity of invasive radio- and chemotherapies to reduce intervention-related long-term morbidity. There are an increasing number of immunotherapy-based treatment strategies including immune checkpoint-inhibitors, oncolytic viruses, CAR-T therapy, and NK cells in recurrent and refractory MBs. Although most trials are in early phase, there is hope for therapeutic breakthroughs for advanced MBs within the next decade.
Keywords: Prognostic biomarker, Risk stratification, Clinical trial, WNT, SHH, Group 3, Group 4, Immunotherapy
Introduction
Medulloblastoma (MB) is the most common malignant childhood tumor of the brain, accounting for ~ 20% of all CNS tumors among children [1]. Current multimodal treatment consisting of surgery, radiation therapy, and chemotherapy allows 60–80% long-term survival [2]. According to a report from the Childhood Cancer Survivor Study comparing long-term mortality and morbidity data, cumulative incidence of all-cause late mortality became significantly lower for MB patients diagnosed in the 1990s compared to the 1970s [3]. Still, more than one-third of patients die within 5 years after diagnosis [1], and the median survival of relapsed/refractory MB is less than 1 year [4]. Each treatment component is responsible for late complications leading to severe, debilitating chronic health conditions [3]. Late mortality remains a significant problem, largely attributable to disease recurrence and secondary malignancies [5]. The current clinical and pathological feature-based risk stratification fails to consider heterogeneity within standard- and high-risk patients. The wide disparities among patient outcomes, the deteriorating quality of life expectations for survivors, and the failure of aggressive, high intensity multimodal therapies to extend life expectancy of patients with recurrent disease illuminate the need for new, more effective treatment approaches.
Integration of molecular data provided by advanced genomics started an exciting refinement of MB classifications [6]: the current consensus agrees upon four distinct molecular subgroups, wingless-activated (WNT-MB), sonic-hedgehog-activated (SHH-MB), and Group 3 and Group 4 MBs [7], each characterized by distinct variety of genetic alterations, transcriptional/methylation profiles, and clinical outcomes [2, 8–10]. Subgroup identity is linked with markedly different survival, although subgroup affiliation does not correlate with response to therapy [11]. Within each primary subgroup, additional molecular markers started to emerge, leading to more refined risk stratifications [2]. Ongoing trials explore personalized treatment strategies for patients harboring specific genetic aberrations.
In this review, we summarize treatment approaches, outline the evolving landscape of molecular classifications, and discuss markers with prognostic relevance. Our main focus is the ongoing transition toward molecular-based risk-adapted strategies in the clinic. We review currently recruiting clinical trials available for both newly diagnosed and recurrent/refractory MB patients. A decent tendency among current trials is the increasing number of targeted and immunotherapy-based approaches, although up till now exclusively for patients with recurrent/refractory MBs.
Clinical classification and current treatment protocols
Current treatment approaches consider two main factors for the selection of postoperative therapy: risk of treatment toxicity and risk of recurrence. Risk of treatment toxicity is especially significant for infants and children younger than 3 years of age. Risk of recurrence is considered to be high in case of disseminated or metastatic disease or suboptimal resection, with a tumor residue ≥ 1.5cm2. Certain histologic subtypes, such as anaplastic and large cell tumors, have also been associated with poor outcome [12]. Three treatment cohorts can be separated based on these criteria: children older than 3 years of age with average-risk disease, children older than 3 years of age with high-risk disease, and infants and children younger than 3 years of age.
Surgical intervention
Surgical goal is maximal safe resection that alleviates increased intracranial pressure and removes as much tumor tissue as possible without causing adverse neurological sequelae, such as cranial nerve deficits or persistent ataxia. Posterior fossa syndrome (cerebellar mutism) still occurs in about one-quarter of patients with MB resection. Development of modern imaging and surgical techniques prompted gross total or near-total resection in most patients [13]. Meanwhile, the magnitude of the extent of resection may not be as significant as earlier thought to be: an inconclusive retrospective systematic review on the extent of resection and disease outcome warrants further evaluation [14]. Prognostic benefits may also be attenuated by molecular subgroups: gross total resection in Group 4 MB patients conferred progression-free survival benefit compared to subtotal resection, especially in the presence of disease dissemination, while the extent of resection was not linked to overall survival (OS) [13].
Radiation therapy (RT)
Following surgery patients are treated with external beam irradiation to the craniospinal axis with an additional boost to the tumor site, with varying radiation doses across risk groups. Most recurrences (50–70%) are local; therefore treatment is limited to the tumor bed compared to the entire posterior fossa which spares critical brain structures [15]. RT is usually initiated 30 days after surgery, since delayed RT administration is associated with lower survival [8, 16]. At the same time, a more recent study identified decreased 5-year OS in patients with RT initiated within 3 weeks after surgery, indicating ample time for healing is also paramount [17].
Patients with average-risk disease are treated with 23.4 Gy craniospinal irradiation (CSI) to the entire brain and spine, with posterior fossa boost for a total dose of 54–55.8 Gy [18–22]. In contrast, high-risk patients receive 36–39.6 Gy CSI with an additional boost to the tumor site, for a total dose of 54–55.8 Gy [21]. Such RT doses are associated with neurocognitive impairment, affecting younger children more negatively [23]. However, reduced-dose neuraxis photon RT results in increased frequency of relapse and decreased survival [24]. In the phase III Children’s Oncology Group ACNS0331 trial, 5.4 Gy reduction in the CSI dose in children between 3 and 7 years of age produced inferior survival compared to standard-dose RT [25]. Deferral of postoperative RT even in the modern era of chemotherapy is associated with poorer OS and is therefore not recommended for children older than 3 years of age [26].
Newer techniques, such as proton radiation therapy and intensity-modulated RT utilized for the posterior fossa boost and spine RT, limit irradiation of the normal tissues with acceptable toxicity and survival similar to conventional RT [27]. Reduced normal tissue dose also relieves post-radiation ototoxicity, leading to preserved hearing and improved quality of life [28, 29]. Nevertheless, additional studies are needed to determine if proton beam RT provides clinically meaningful long-term cognitive sparing compared to photon beam RT protocols [30].
Chemotherapy
Following postsurgical RT, chemotherapy became accepted as a standard treatment as it improves survival and reduces RT-related adverse effects, especially in younger children. Numerous risk-adapted multimodal treatment protocols have been tested in clinical trials. A randomized, multicenter trial (HIT’91) compared the long-term effects of a neoadjuvant (“sandwich”) chemotherapy (incorporating ifosfamide, etoposide, high-dose methotrexate, cisplatin, and cytarabine, followed by CSI and maintenance chemotherapy) versus “maintenance” chemotherapy after radiation (consisting of vincristine complementary to RT and eight cycles with CCNU, vincristine and cisplatin 6 weeks after RT), adapted from the “Packer protocol.” The “maintenance” regimen resulted in significantly higher 10-year OS compared to the “sandwich” approach among patients with M0 (91% vs. 62%) or M1 (70% vs. 34%) disease [31, 32].
The phase III Children’s Oncology Group (COG) study adopted a weekly vincristine treatment during RT, followed by eight cycles of chemotherapy including vincristine and cisplatin plus CCNU, or vincristine and cisplatin plus cyclophosphamide. The strategy leads to 76% and 81% 10-year event-free and overall survival, respectively, in average-risk patients, with no differences in efficacy between the two chemotherapy regimens [22, 33], producing at least as good outcome as previous, more intensive treatment protocols.
The current treatment approach includes risk-adapted RT and four cycles of cyclophosphamide-based, dose-intensive chemotherapy (incorporating cisplatin, vincristine, and cyclophosphamide), followed by stem cell or bone marrow rescue. This regimen produced 85% 5-year survival (95% CI 75–94) in the average-risk MB population and 70% 5-year survival (95% 54–84) in the high-risk population [21]. Histological subtype also correlated with 5-year event-free survival and was lowest (57%) for large cell anaplastic tumors [21].
Additional ongoing clinical trials test the feasibility of alternative treatment regimes in newly diagnosed MBs. The recruiting SIOP PNET 5 MB phase II/III trial (NCT02066220) evaluates the outcome in patients with non-WNT “standard-risk” biological profiles, defined by nuclear beta-catenin immune-negativity, after concurrent carboplatin and radiotherapy, followed by eight cycles of maintenance chemotherapy (Table 1).
Table 1.
List of ongoing clinical trials currently recruiting newly diagnosed medulloblastoma patients
| ID | Phase | Treatment | Treatment arms | Subgroup | Risk | Eligible age cohort | Allocation | Primary outcome measure | Estimated primary completion date |
|---|---|---|---|---|---|---|---|---|---|
| NCT01878617 | II | Treatment stratification based on both clinical risk (low-, standard-, intermediate-, or high-risk) and molecular subtype (WNT, SHH, or non-WN/non-SHH) | Low-risk (stratum W1): reduced-dose craniospinal irradiation, lower dose of cyclophosphamide | WNT (strata W): positive for WNT biomarkers | All risk categories | 3 years to 39 years | Non-randomized | Progression-free survival | 2023 |
| atypical (W2): standard-dose craniospinal radiation, standard chemotherapy (cisplatin, vincristine, cyclophosphamide) | Change in VO2 peak values after aerobic training intervention; change in spatial span backward standard score | ||||||||
| High-risk (W3): high does craniospinal radiation, standard chemotherapy | |||||||||
| Standard-risk (S1): standard-dose craniospinal radiation, chemotherapy, vismodegib maintenance therapy for skeletally | SHH (strata S): positive for SHH biomarkers | ||||||||
| High-risk (S2): high-dose craniospinal radiation, chemotherapy, vismodegib maintenance therapy for skeletally mature patients | |||||||||
| Standard-risk (N1): standard-dose craniospinal radiation, chemotherapy | Non-WNT/non-SHH, failed, or indeterminate (Strata N): negative for WNT and SHH biomarkers for results are indeterminable | Progression-free survival | |||||||
| intermediate risk (N2): standard-dose craniospinal radiation, standard chemotherapy (cisplatin, vincristine, cyclophosphamide) for four cycles intermixed with an additional three cycles of chemotherapy with pemetrexed and gemcitabine | Change in VO2 peak values after aerobic training intervention; change in spatial span backward standard score | ||||||||
| High-risk (N3): high-dose craniospinal radiation, standard chemotherapy with an additional three cycles of chemotherapy with pemetrexed and gemcitabine | |||||||||
| NCT01857453 | II | Reduced irradiation compensated by chemotherapy | Carboplatin + etoposide-based chemotherapy followed by radiation therapy with 24 Gy on the in toto neuro axis and 54 Gy on the post-operative bed | Standard-risk | 18 years to 70 years | Single group assignment | Survival without disease at 1 year | 2018 | |
| NCT02025881 | I/II | Sequential high-dose chemotherapy | Carboplatin + etoposide then thiotepa then cyclophosphamide + busilvex | High-risk | Up to 5 years | Single group assignment | Phase I- maximum tolerated dose; phase II- event- free survival up to 3 years | 2018 | |
| NCT02875314 | IV | Induction chemotherapy followed by either a single cycle of three tandem cycles | Single cycle (thiotepa, etoposide, and carboplatin for 6 days) | Non-WNT/non-SHH | High-risk | Up to 10 years | Randomized | Event-free survival, overall survival | 2020 |
| three tandem cycles (thiotepa and carboplatin over 2 days, three cycles) | |||||||||
| NCT02724579 | II | Reduced craniospinal radiotherapy | Reduced craniospinal radiotherapy (18 Gy) with a limited target volume boost to the tumor bed of 36 Gy for a total of 54 Gy and reduced chemotherapy (no vincristine during chemotherapy and reduced-dose maintenance chemotherapy) | WNT | Standard-risk | 3 years to 21 years | Single group assignment | Progression-free survival | 2025 |
| NCT02066220 | II/III | Risk-specific radio- and chemotherapy | Low-risk: radiotherapy and reduced-intensity maintenance chemotherapy consisting of 3 courses of cisplatin, CCNU, and vincristine alternating with three courses of cyclophosphamide and vincristine | ẞ-catenin nuclear immunopositivity (non-metastatic) | Low- and standard- risk | 3 years to 21 years | randomized | 3-year event-free survival | 2024 |
| Standard-risk: radiotherapy with carboplatin or radiotherapy without carboplatin and maintenance chemotherapy consisting of four courses of cisplatin, CCNU, and vincristine alternating with four courses of cyclophosphamide and vincristine | ẞ-catenin nuclear immunonegativity (non-metastatic) |
Abbreviations: CSI, craniospinal radiation; PFS, progression-free survival; OS, overall survival; EFS, event-free survival; DFS, disease-free survival
Up to 30% of MB patients are diagnosed with a metastatic disease [34]. Despite high intensity RT and chemotherapy, the risk of recurrence and death remains elevated. A Children’s Oncology Group Phase I/II trial for metastatic patients administered 36 Gy CSI with boosts to the sites of disease, complemented with carboplatin along with vincristine during radiation, followed by six maintenance cycles of cyclophosphamide and vincristine with or without cisplatin. The regimen without cisplatin resulted in 82% 5-year survival, compared to 68% in the cisplatin arm [35]. Another approach for high-risk patients included a reduced-dose CSI (23.4 to 30.6 Gy) complemented with tandem high-dose chemotherapy (2 cycles pre- and 4 cycles post RT) and autologous stem cell transplantation. The treatment resulted in 70% 5-year event-free and 74% overall survival rates [36], although sample size was low and longer follow-up is needed to evaluate the potential long-term risks of high-dose chemotherapy, especially as chemotherapy may aggravate neurological adverse effects [37]. The HIT’2000 trial for patients with gross leptomeningeal dissemination (M2/3) adapted an intensified “sandwich”-regimen, including strengthened induction chemotherapy (comprising two cycles of intravenous cyclophosphamide, vincristine, methotrexate, carboplatin, etoposide, and concomitant intraventricular methotrexate), hyperfractionated CSI, and four cycles of maintenance chemotherapy (consisting of cisplatin, CCNU, and vincristine). The trial resulted in superior survival rates compared to the preceding HIT’91 trial: the 5-year OS reached 74% (95% CI 66–82%), with histology and nonresponse to the first chemotherapy cycle as independent risk factors [38]. Survival was associated with molecular subgroup identity and the presence of MYCC/MYCN amplifications, suggesting that these may be useful biomarkers in future treatment stratifications [38].
Infants and children under the age of 3 are preferably treated by surgery and chemotherapy alone due to high-risk of radiation-induced morbidity [39]. The CCG-99703 protocol consists of high-dose chemotherapy (vincristine, cyclophosphamide, etoposide, and cisplatin) followed by autologous hematopoietic cell rescue, while the HIT-SKK’92 protocol endorses systemic chemotherapy and intraventricular therapy (intravenous and intraventricular methotrexate, vincristine, cyclophosphamide, and carboplatin) [40, 41]. HIT-SKK’92 led to better outcomes in patients with gross total resection and without metastases compared to patients with residual or metastatic disease [41–43]. Delay of radiation therapy may be particularly favorable in young children with desmoplastic/extensive nodular histology [44], and sequential high-dose chemotherapy following the CCG-99703 protocol produced an excellent outcome for SHH-MB patients with classical histology [39].
Novel protocols are being evaluated to circumvent the debilitating complications linked to radiation therapy by extending the age range of young patients (Table 1). A phase II (NCT02025881) open-label, non-randomized trial focuses on children under the age of 5 with newly diagnosed high-risk MBs and investigates the efficacy of a sequential high-dose chemotherapy without radiotherapy with stem cell support. Risk assignment is based on histology (large cell/anaplastic (LCA) or other unfavorable), dissemination, and the presence of MYC and MYCN amplifications, and the chemotherapy regimen includes carboplatin + etoposide, then thiotepa, then cyclophosphamide, and busilvex. The study includes a phase I dose-finding part to determine the dose of cyclophosphamide that can be given in combination with busilvex. The phase IV HeadStart4 trial (NCT02875314) focuses on newly diagnosed high-risk children (up to 10 years of age) mainly consisting of non-WNT/non-SHH-MBs, and evaluates the efficacy of a high intensity induction therapy (including vincristine, cisplatin, cyclophosphamide, etoposide and high-dose methotrexate) followed by either a single cycle (carboplatin, thiotepa, etoposide) or three tandem cycles (carboplatin, thiotepa) of consolidation marrow-ablative chemotherapy with autologous hematopoietic progenitor cell rescue. Risk assessment is based on clinical features such as age (all MBs under the age of 6), histology, and stage (high disease stage with classic or LCA histology between the ages of 6 and 10).
In adults, MBs are rare, accounting for < 1% of all CNS-tumors, and most adult patients belong to the standard clinical risk group. Due to the relatively low incidence of adult MBs, there are no randomized clinical trials upon which to base treatment recommendations. After maximal safe resection, adult patients are treated with normofractionated CSI of 30–36 Gy followed by a tumor bed boost to 54–55.8 Gy and occasional chemotherapy, particularly for high-risk disease, with unknown outcomes [45, 46]. However, such conventional radiation therapy is associated with progressive neurotoxicity, leading to the degradation of the quality of life. An ongoing phase II trial (NCT01857453) investigates the effects of reduced irradiation compensated by carboplatin- and etoposide-based chemotherapy in newly diagnosed, clinically standard-risk adult patients (ages 18 to 70) (Table 1). The study also aims to assess the relevance of prognostic biomarkers used in the pediatric population for older patients.
Molecular classifications and subgroup-specific molecular alterations
Given the wide variety of responses to standard of care treatment approaches, the current challenge is to identify patients for whom reduced intervention may boost cure rates while alleviating long-term adverse sequelae or alternatively to identify those who are incurable with paradigm treatments and whom participation in clinical trials would be recommended. Thus, recognizing molecular subgroups provides an opportunity to personalize therapy and avoid over- or under-treatment.
An early genomics study investigating gene expression-based clustering partitioned MB into separate subgroups, each with different histologic, molecular and clinical profiles [47], and the results were corroborated by subsequent analyses with extended sample sizes [12, 48–50]. Four main molecular subgroups were defined [7], and the new subclassification was integrated into the revised WHO system published in 2016 [6]. WNT- and SHH-activated MBs are clearly separable entities from the other subgroups, while no dominant signaling pathway alterations were identified in Group 3 and Group 4 MBs. The latter ones are more related to each other than to the other MBs and therefore appear as non-WNT/non-SHH in the revised WHO classification [6]. The subgroup assignment is still provisory and flexible to further alterations, as Group 3 and 4 MBs encompass a heterogeneous population at both the molecular and clinical levels [13, 49, 51]. Subgroup identity is highly prognostic with markedly different outcomes across the four principal subgroups [11], although survival also strongly depends on histology, presence of metastases, and molecular abnormalities [7, 12, 52, 53].
WNT-activated MBs
WNT-MBs are the least common, accounting for about 10% of all MB patients (Fig. 1). More than 90% of tumors display classic histology, and metastasis is rare with ~ 9% frequency at diagnosis [49, 52]. WNT-MBs are typically absent in infants and are found among older children (median age of 10–12 years), with almost equal ratio of males and females [7, 54]. Prognosis under 16 years of age is usually excellent, with more than 90% 5-year event-free survival [55]. The first European prospective study of MB biomarkers investigating non-metastatic, clinically defined standard-risk tumor samples validated the good prognosis associated with WNT-activated MBs. Higher frequency of relapses were observed in patients older than 16 years of age at diagnosis and in those with delayed radiotherapy, indicating that radiation is an important treatment component [56]. Outcome in adult patients (over 16 years of age) requires further investigations [57, 58]. A recent large-scale study integrating gene expression and methylation data divided WNT-activated MBs into two subtypes: WNT α consists predominantly of children with chromosome 6 monosomy and WNT β represents adults mainly with diploid chromosome 6. The two subtypes harbor similar survival rates, although prognosis in adults is worse and underlying pathway activations are different [58, 59].
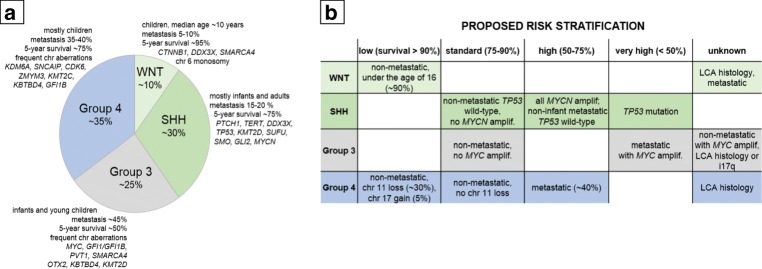
Fig 1.
(a) The four molecular subgroups of MBs according to the current consensus and (b) the proposed risk stratification, based on [2]. WNT-activated (WNT-MB), SHH-activated (SHH-MB), and Group 3 and Group 4 MBs are distinguished by origin, demographics, genetic and molecular alterations, and clinical outcome
Stabilizing mutation of the CTNNB1 gene is the most common genetic alteration, present in 85–90% of all WNT-activated MBs [8, 60], frequently associated with chromosome 6 monosomy, occurring in about 70–80% of patients [8, 47, 61]. In the large-scale MAGIC study, about 5% of WNT-activated MBs with typical WNT-signatures had neither CTNNB1 mutations nor chromosome 6 monosomy [62]. Thus, diagnosis solely based on these two events may miss up to 10–15% of WNT-activated MBs [8]. CTNNB1 mutations frequently occur with mutations of chromatin remodeling genes (CREBBP, TRRAP, and MED13) and/or subunits of the SWI/SNF nucleosome-remodeling complex (SMARCA4, ARID1A, and ARID2) suggesting that besides activation of CTNNB1, aberrant chromatin regulation may also be necessary for WNT-MB [8, 60, 61]. Half of β-catenin mutated WNT-activated MBs harbor concurrent DDX3X mutations [61], but these mutations are not subgroup-specific: 10–20% of SHH and 3% of Group 3 tumors also carry DDX3X mutations [60]. DDX3X participates in multiple cellular processes, including mRNA splicing, export, and translation [63], and its mutations are predicted to increase the proliferation rate of the lower rhombic lip progenitors [60]. DDX3X is located on the X chromosome, and not unexpectedly its mutations have a strong male predominance [64]. The relatively frequent CSNK2B (wingless signaling pathway) and EPHA7 (cellular signaling) mutations are additional clinically actionable targets [8]. Although TP53 mutations are also relatively frequent, these have no prognostic significance in WNT-MBs [8, 61, 65].
The rare germline mutation of APC on chromosome 5 as part of the inherited Turcot syndrome contributes to the development of familial WNT-MBs. APC mutations are coupled with distinct gene expression signatures, suggesting that CTNNB1 and APC mutations are mutually exclusive and molecularly distinct [8]. Rare germline lesions in anaplastic lymphoma kinase gene (ALK) have recently been identified in WNT-activated MBs [66], while other alternative mechanisms, such as mutations of CDH1, may promote tumor development [60]. There are no frequent, targetable somatic copy number aberrations in WNT-MBs [8, 62].
SHH-activated MBs
SHH-MBs account for ~ 30% of all MBs with an intermediate prognosis (5-year OS of 70%) [49] and are most frequently found in infants and adults, with relatively fewer childhood cases [8, 10, 52]. Age at diagnosis is associated with distinct molecular and clinical categories [57, 67]. The majority of SHH-MBs are nodular/desmoplastic but can also have a classic or LCA histology, the latter especially frequent among children [54, 68]. The nodular desmoplastic subtype predicts increased survival in infants [44, 69, 70].
Over 95% of SHH-MBs contain at least one driver event. However, the types of mutations are highly variable [8, 68]. SHH-MBs have the highest, 14–20% prevalence of destructive germline mutations among all MBs. Li-Fraumeni syndrome is linked to hereditary TP53 mutations (~ 20% of all SHH-MB), most frequent among children aged 5–16, predisposing to multiple familial cancers and coupled with a low survival [71]. Li-Fraumeni syndrome-associated SHH-MBs have an exceptionally high mutational rate [72], and radiation therapy can accelerate tumor growth [73]. Gorlin syndrome is associated with mutations affecting PTCH1 and SUFU genes of the SHH-pathway [74], present in 21% of all infant SHH-MBs, where radiation also increases the likelihood of secondary, mostly skin cancers [75].
Activating mutations almost permanently involve the SHH-signaling pathway [68], including the mutually exclusive and age-group specific PTCH1 (~ 43%), SUFU (~ 10%), and SMO (~ 9%) mutations [8, 60, 68, 72]. SUFU and SFO mutations are enriched in infants (0–4 years) and adults, respectively, and the presence of PTCH1 mutations is roughly equal in all age groups [68]. In children, TP53 mutations are mutually exclusive with PTCH1 mutations but frequently co-occur with GLI2 and MYCN amplifications, essential regulators of transcription [8]. Loss of chromosome 17p loss is also frequent [68], as are TP53 mutations that interestingly do not confer a survival disadvantage [72]. TP53 mutational status separates SHH-MBs with distinct outcomes: tumors with TP53-wildtype are more frequent among adults and young children and are linked to favorable prognosis (81% 5-year OS). In contrast, TP53 mutations are typical among older children and are associated with dismal outcome (40% 5-year OS). TP53 mutations account for two-thirds of deaths in children older than 5 years. In SHH-MB, more than half of TP53-mutantion are germline [65]. Mutations of some genes are almost exclusively specific to the adult subgroup, including TERT promoter mutations that drive telomerase activity [76] or recurrent mutations of the PI3K/AKT/mTOR pathway [68]. Additional subgroup-specific molecular alterations affect chromatin modulation, histone acetyltransferase, or nuclear receptor corepressor complexes [77]. High expression often cannot be traced to specific mutations or chromosome aberrations; like in the case of cMET that is among the most frequently hypo- or hypermethylated genes in MBs [78].
Group 3 and Group 4 MBs
Group 3 MBs represent approximately 25% of all cases (Fig. 1) with a peak of diagnosis between ages 3 and 5 years and is the deadliest of all MBs, with a 58% 5-year OS in children and a 45% in infants [9, 13, 52]. Male to female ratio is about 2:1 [12]. Group 3 MBs are mostly classical, although 40% harbor LCA histology, assigning most LCA tumors to this subgroup [12]. The aggregation of multiple adverse circumstances, such as young age coupled with metastatic spread (up to 50% of patients), the presence of MYC amplifications, and high rate of LCA histology, all contribute to the poor prognosis. MYC-amplified tumors confer an especially short survival with only 20% of patients surviving 5 years post-diagnosis [49, 79]. Absence of metastatic spread does not necessarily reflect good prognosis [67]; thus, standard-risk children with Group 3 disease may face under-treatment [52].
Group 4 is the most prevalent (~ 35%, Fig. 1) and also the least understood subgroup [7, 52] including about 45% of all childhood and 25% of adult cases with an overall intermediate prognosis (5-year overall survival 75–90%) [7]. The prevalence is three times higher in males than in females [7, 52]. Outcome is excellent in patients with chromosome 11 loss where survival exceeds 90% [2]. Nevertheless, 30–40% of Group 4 MB patients are diagnosed with metastases and treated for high-risk disease, with 5-year survival as low as 60% [2, 11, 52]. Adults with Group 4 MBs have a significantly worse prognosis compared to the SHH- or WNT-activated subtypes [58].
Group 3 and Group 4 MBs are genetically heterogeneous, not driven by well-defined, constitutively activated signaling pathways, and rarely have damaging germline mutations in known cancer predisposition genes [71]. Tetraploidy is a recurrent early genetic event, leading to an increased number of large-scale copy number gains [72]. The most frequent cytogenetic alterations in Group 3 MBs include the loss of 17p (55%) along with loss of 16q (42%), 10q (43%), and 9q (21%) and the gain of 17q (in 58% of samples), 7 (39%), and 1q (41%) [52]. Somatic MYC (17% in Group 3) and MYCN (6% Group 4) amplifications are frequent driver events [62], and high MYC levels are associated with meager outcome [49, 80]. Other recurrent genetic events in Group 3 MBs include OTX2 and EZH2 amplifications, SMARCA4 mutations, GFI1 enhancer activation, copy number alterations in TGFβ pathway genes, and mutations in Notch signaling genes [81]. Tetraploidy is also present in approximately 40% of Group 4 tumors with unknown prognostic significance [72]. Isochromosome 17q occurs in about 80% of Group 4 tumors, but it does not confer poor prognosis. Other major recurrent genetic events in Group 4 MBs include 8p loss (41%), 10q loss (15%), aberrations of 11p and 18q, gain of chromosome 7 (47%), complete loss of one X chromosome in about 80% of females [49, 52, 67, 82], amplifications of MYCN and CDK6, SNCAIP duplications, and mutations in genes responsible for chromatin modification, such as KDM6A [81].
Emerging risk stratification models
A 2015 consensus conference held in Heidelberg incorporated prognostic molecular markers and suggested a more refined risk stratification for non-infant tumors (ages 3–17). The proposed four categories include low, standard, high, and very high-risk MBs [2] (Fig. 1). The low-risk (survival > 90%) group slots in non-metastatic patients under the age of 16 with WNT-MBs [2], possibly including patients with incomplete resections [13]. Patients over 16 years of age may not be low-risk, and recommendations for patients harboring metastatic disease or LCA histology are unclear. Non-metastatic Group 4 patients with chromosome 11 loss (about one-third) and/or whole gain of chromosome 17 (about 5%) also qualify as low-risk [11]. The standard-risk (survival 75–90%) population includes non-metastatic SHH patients excluding TP53-mutated or MYCN amplified tumors, non-metastatic Group 3 MBs with no MYC amplification, and non-metastatic Group 4 MBs without chromosome 11 loss [11, 51, 65]. The high-risk (50–75% survival) cohort consists of all MYCN-amplified SHH-activated MBs (regardless of the metastatic status): TP53 wild-type, non-infant metastatic SHH-activated and metastatic Group 4 MBs [11]. The very high-risk (< 50% survival) population includes SHH patients with TP53 mutations and metastatic Group 3 MBs with MYC amplifications [2, 11, 65]. The risk evaluation of non-metastatic but MYC-amplified Group 3 tumors with an LCA histology or isochromosome 17q or Group 4 MBs with anaplastic histology requires further clarifications [2].
Molecular classification of MBs is constantly evolving, and additional stratification schemes started to emerge based on genome-wide molecular approaches. A large-scale study integrating gene expression and methylation data by similarity network fusion divided each subgroup into further biologically and clinically relevant subtypes. The resulting 12 subtypes (2 in WNT, 4 in SHH, and 3 in both Group 3 and Group 4) are suggested to be different on the molecular, clinical, and prognostic levels [59]. Another study utilizing methylation signatures and unsupervised class discovery distinguished seven robust subtypes, all predictive of clinical outcome. The analysis splits current subgroups, except WNT, into further subtypes: SHH by an age-dependent manner into infant (< 4.3 years) and children cohorts while both Group 3 and Group 4 into high-risk (HR) and low-risk (LR) categories with dramatically different survival rates. Within each subtype, secondary molecular characteristics strongly influenced prognosis and resulted in four different risk categories from favorable to very high-risk. The overall model is suggested to outperform other MB risk stratifications [16]. Novel subtypes with specific genetic signatures were identified in another large-scale study utilizing somatic landscape and epigenetic analyses [8].
The discrepancies across classification schemes stem from diverse patient populations (children only or all ages), types of data utilized, and clustering methods. Still, the emerging differences hold promise for improved stratification and genotype-specific treatment. Nevertheless, well-planned prospective clinical trials are inevitable to reach a consensus across stratification models and to identify appropriate biomarkers.
Molecular subgroup-specific treatment approaches
WNT-activated MBs
WNT-MBs contain a leaky vascular endothelium that disrupts the blood-brain barrier (BBB) integrity. The lack of functional BBB increases penetration of systemic chemotherapy and contributes to the excellent prognosis seen in WNT-MB patients even in the presence of metastases [83]. The Wnt pathway has a crucial role in various developmental processes, including tissue regeneration and bone formation [84]. Targeting the Wnt pathway would interfere with such physiological mechanisms and could also eliminate the advantageous chemosensitivity of WNT-activated MBs. Consequently, targeted therapies against WNT-MBs are not being developed. Instead, current therapy de-escalation for low- or standard-risk WNT-MBs is tested by several clinical trials (Table 1). Trial NCT01878617 utilizes molecular subgroup allocation and clinical risk category at treatment selection and investigates therapy de-escalation for low-risk WNT-activated MBs. Another phase II trial (NCT02724579) studies the effects of reduced CSI and chemotherapy (no vincristine during radiotherapy and reduced-dose maintenance chemotherapy) on average-risk WNT-activated MBs defined by positive nuclear beta-catenin expression (by IHC), CTNNB1 mutations, and absence of MYC and MYCN amplifications (by FISH).
The SIOP PNET 5 phase II/III trial (NCT02066220) evaluates the efficacy of risk-dependent treatment adjustments. The aim is to confirm high event-free survival in patients with a low-risk profile defined by non-metastatic MB with total and near-total tumor resection, absence of MYC and MYCN amplifications and LCA histology, and nuclear beta-catenin immune positivity. Low-risk (WNT-activated) patients receive radiotherapy with a dose of 54 Gy to the primary tumor and 18.0 Gy to the craniospinal axis without carboplatin, followed by six cycles of reduced-intensity maintenance chemotherapy (consisting of three courses of cisplatin, CCNU, and vincristine alternating with three courses of cyclophosphamide and vincristine).
SHH-activated MBs
Inhibition of smoothened (SMO) offers avenues for a targeted subgroup-specific treatment strategy for SHH-activated MBs. SMO inhibition blocks downstream signaling, Gli translocation, and activation of Hedgehog target genes [85]. Most adult SHH-MB patients (80%) carry PTCH1 or SMO mutations. Vismodegib (GDC-0449), a small molecule inhibitor of SMO, demonstrated particular efficacy against recurrent/refractory MBs [86, 87]. Nevertheless frequent mutations downstream of SMO, such as mutations affecting SUFU or GLI1, render most infants and children resistant to targeted vismodegib therapy [68]. Moreover, SMO inhibition hinders normal bone and teeth development in pediatric patients causing growth plate fusions that persist long after therapy cessation [88]. Preclinical efforts seeking targeted therapies against SHH-MBs also focus at epigenetic treatments with BET bromodomain inhibitors, inhibition of the G2/M regulators AURK and PLK, cMET inhibitors, strategies targeting stem-like cells, and interventions utilizing immunotherapies [77].
The currently recruiting phase II (NCT01878617) trial investigates the feasibility and toxicity of oral vismodegib maintenance therapy after conventional chemotherapy for standard-risk or high-risk newly diagnosed MBs in skeletally mature SHH-activated patients (Table 1). For recurrent/refractory SHH-activated MBs, further treatments and drug combinations are available (Table 2). A phase I trial organized by the St. Jude Children’s Research Hospital (NCT03434262) investigates subgroup-specific doublet combinations including the CDK4/6 inhibitor ribociclib and SMO inhibitor sonidegib (LDE225) for adult SHH-activated patients, with copy number loss of 9q or PTCH1 mutations and ribociclib and MEK-inhibitor trametinib for WNT- and SHH-activated MBs.
Table 2.
Ongoing clinical trials currently recruiting patients with recurrent/refractory/progressive medulloblastomas
| ID | Phase | Treatment | Subgroup | Eligible age cohort | Estimated enrollment | Estimated primary completion date | Responsible party |
|---|---|---|---|---|---|---|---|
| NCT00089245 | I | Intrathecal radioimmunotherapy using I-8H9 | 8H9 reactive malignancy confirmed by IHC | Child, adult, older adults | 120 | 2018 | Y-mAbs Therapeutics |
| NCT02502708 | I | Indoximod, an inhibitor of the immune “checkpoint” pathway indoleamine 2,3-dioxygenase (IDO), in combination with temozolomide | 3 years to 21 years | 115 | 2019 | NewLink Genetics Corporation | |
| NCT02684071 | II | Methotrexate and chemotherapy (topotecan and cyclophosphamide) | up to 21 years | 10 | 2019 | Ziad Khatib, Nicklaus Children’s Hospital f/k/a Miami Children’s Hospital | |
| NCT02905110 | Early phase I | Simultaneous methotrexate/etoposide infusion | Posterior fossa tumors | 1 year to 80 years | 10 | 2020 | David Ilan Sandberg, The University of Texas Health Science Center, Houston |
| NCT01661400 | Early phase I | Metronomic cyclophosphamide or thalidomide | 6 months to 21 years | 12 | 2020 | Washington University School of Medicine | |
| NCT02962167 | I | Modified measles virus (MV-NIS) | 12 months to 39 years | 46 | 2020 | Sabine Mueller, MD, PhD, University of California, San Francisco | |
| NCT03043391 | I | Oncolytic poliovirus (PV) immunotherapy with PVSRIPO | 12 years to 21 years | 12 | 2020 | Istari Oncology, Inc. | |
| NCT02271711 | I | Autologous ex vivo-expanded NK cells | up to 21 years | 24 | 2020 | MD Anderson Cancer Center | |
| NCT02359565 | I | Pembrolizumab | 1 year to 29 years | 110 | 2020 | National Cancer Institute (NCI) | |
| NCT03389802 | I | APX005M, a humanized IgG1κ mAb that binds to CD40 | 1 year to 21 years | 45 | 2020 | Pediatric Brain Tumor Consortium | |
| NCT03299309 | I | Cytomegalovirus (CMV)-specific peptide vaccine (PEP-CMV) | 3 years to 35 years | 30 | 2020 | Gary Archer Ph.D., Duke University | |
| NCT03734913 | I | ZSP1602, a SMO protein inhibitor | 18 years to 75 years | 65 | 2020 | Guangdong Zhongsheng Pharmaceutical Co., Ltd. | |
| NCT03598244 | I | Savolitinib, a small molecule inhibitor of c-Met | 6 years to 21 years | 36 | 2020 | National Cancer Institute (NCI) | |
| NCT03387020 | I | Ribociclib (inhibitor of cyclin D1/CDK4 and CDK6) in combination with everolimus | 1 year to 21 years | 45 | 2020 | Pediatric Brain Tumor Consortium | |
| NCT03173950 | II | Nivolumab | 18 years to 99 years | 180 | 2020 | National Institutes of Health Clinical Center (CC) (National Cancer Institute (NCI)) | |
| NCT03273712 | II | 90Y-DOTA-tyr3-Octreotide for somatostatin receptor positive tumors | Tumors expressing somatostatin receptors | 6 months to 90 years | 46 | 2020 | Sue O’Dorisio, University of Iowa |
| NCT02644291 | I | Mebendazole, an antihelmintic agent | 1 year to 21 years | 21 | 2021 | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | |
| NCT03500991 | I | HER2-Specific CAR T-cell locoregional immunotherapy | 1 year to 26 years | 36 | 2021 | Julie Park, Seattle Children’s Hospital | |
| NCT02095132 | I/II | Adavosertib (MK-1775), a small molecule inhibitor of the tyrosine kinase WEE1 and irinotecan hydrochloride | 1 year to 21 years | 154 | 2021 | National Cancer Institute (NCI) | |
| NCT01356290 | II | Multidrug antiangiogenic approach | up to 19 years | 40 | 2021 | Andreas Peyrl, Medical University of Vienna | |
| NCT03911388 | I | G207, an oncolytic herpes simplex virus-1 (HSV) | 3 years to 18 years | 15 | 2022 | Gregory K. Friedman, MD, University of Alabama at Birmingham | |
| NCT03638167 | I | EGFR806-specific CAR T-cell locoregional immunotherapy | 1 year to 26 years | 36 | 2022 | Julie Park, Seattle Children’s Hospital | |
| NCT03893487 | Early phase I | Fimepinostat (CUDC-907), a small molecule inhibitor of histone deacetylase and PI3 kinase | 3 years to 39 years | 30 | 2022 | Sabine Mueller, MD, PhD, University of California, San Francisco | |
| NCT03709680 | I | Palbociclib in combination with temozolomide and irinotecan | 2 years to 20 years | 100 | 2022 | Pfizer | |
| NCT03904862 | I/II | CX-4945 (silmitasertib sodium), small molecule inhibitor of casein kinase II (CK2) | SHH | 3 years and older | 60 | 2022 | Pediatric Brain Tumor Consortium |
| NCT02748135 | I/II | TB-403 (humanized monoclonal antibody against placental growth factor (PlGF) | 6 months to 18 years | 36 | 2022 | Oncurious NV | |
| NCT03936465 | I | BMS-986158, a bromodomain (BRD) and extra-terminal domain (BET) inhibitor | Predicted increased sensitivity to BET inhibition: MYC or MYCN amplification or high copy number gain, MYC or MYCN translocation, BRD4 or BRD3 translocation | 1 year to 21 years | 34 | 2022 | Steven DuBois, Dana-Farber Cancer Institute |
| NCT02650401 | I/II | Entrectinib (RXDX-101), a TRKA/B/C, ROS1, and ALK inhibitor | CNS tumors harboring- NTRK1/2/3, ROS1, ALK molecular alterations, including gene fusions | up to 22 years | 65 | 2023 | Hoffmann-La Roche |
| NCT04049669 | II | Indoximod in combination with radiation and chemotherapy | 3 to 21 years | 140 | 2024 | Theodore S. Johnson, Augusta University | |
| NCT03210714 | II | JNJ-42756493 (erdafitinib), an oral pan-FGFR inhibitor | Mutations in the FGFR1/2/3/4 pathway | 12 months to 21 years | 49 | 2024 | National Cancer Institute (NCI) |
| NCT03213678 | II | Ly3023414 (samotolisib), a PI3K/mTOR inhibitor | TSC loss of function mutations, PI3K/MTOR activating mutations | 12 months to 21 years | 144 | 2024 | National Cancer Institute (NCI) |
| NCT03213704 | II | LOXO-101 (larotrectinib) | NTRK fusions | 12 months to 21 years | 49 | 2024 | National Cancer Institute (NCI) |
| NCT03213665 | II | Tazemetostat, a small molecule EZH2 inhibitor | EZH2, SMARCB1, or SMARCA4 gene mutations | 12 months to 21 years | 49 | 2024 | National Cancer Institute (NCI) |
| NCT03233204 | II | Olaparib | Defects in deoxyribonucleic acid (DNA) damage repair genes | 12 months to 21 years | 49 | 2024 | National Cancer Institute (NCI) |
| NCT04023669 | I | Prexasertib (LY2606368), a molecularly targeted CHK1/2 inhibitor | Group3/Group4; SHH; indeterminate | 1 year to 24 years | 100 | 2025 | St. Jude Children’s Research Hospital |
| NCT03434262 | I | Ribociclib and gemcitabine | Group3/Group4 | 1 year to 39 years | 108 | 2025 | St. Jude Children’s Research Hospital |
| Ribociclib and trametinib | SHH/WNT | 1 year to 39 years | 108 | 2025 | St. Jude Children’s Research Hospital | ||
| Ribociclib and sonidegib | SHH | 1 year to 39 years | 108 | 2025 | St. Jude Children’s Research Hospital | ||
| NCT03526250 | II | Palbociclib | Rb positive, or mutations in cell cycle genes | 12 months to 21 years | 49 | 2025 | National Cancer Institute (NCI) |
| NCT03155620 | II | Treatment directed by mutation carrier screening | Based on results of molecular screening | 12 months to 21 years | 1000 | 2027 | National Cancer Institute (NCI) |
The Pediatric Brain Tumor Consortium Phase I/ II trial NCT03904862 evaluates the effects of CX-4945, silmitasertib sodium, a small molecule inhibitor of Casein kinase II (CK2) in skeletally immature (phase I) and skeletally mature (phase II), SHH-activated recurrent/refractory MB patients, where subgroup identity is confirmed by a CLIA certified methylation-based assay. The St. Jude ELIOT (NCT04023669) phase I trial evaluates prexasertib (LY2606368), a targeted CHK1/2 inhibitor in combination with cyclophosphamide for recurrent/refractory SHH-activated or Group 3/Group 4 MBs. Another trial, NCT03734913, explores ZSP1602, a SMO protein inhibitor, designed for adult patients with advanced MBs, regardless of SMO or Gli1 alteration status and molecular subgroup identity.
Group 3/Group 4 MBs
The limited understanding of tumorigenesis mechanisms hampers the development of targeted treatment strategies for Group 3 and Group 4 MBs. For newly diagnosed patients, the NCT01878617 trial offers risk-adapted treatment strategy: intermediate- and high-risk patients are exposed to new chemotherapy agents (pemetrexed and gemcitabine) after standard chemotherapy and risk-adapted radiotherapy (Table 1).
For recurrent and refractory MB patients, two trials offer treatments based on molecular subgrouping (Table 2). In NCT04023669, prexasertib (LY2606368), a targeted CHK1/2 inhibitor, is administered in combination with cyclophosphamide for Group 3, Group 4, and SHH-activated MBs or in combination with gemcitabine for Group 3/Group 4 MBs. The trial NCT03434262 investigates the combination of the cyclin-dependent kinase inhibitor ribociclib and gemcitabine for Group 3/Group 4 MBs.
In Group 3 MBs, preclinical studies focus at the inhibition of PI3K and mTOR signaling pathways, evaluate the synergistic activity between histone deacetylase inhibitors and PI3K inhibitors, assess the efficacy of CDK, PRKDC or BET bromodomain inhibitors, and test the effectiveness of anti-vascularization therapies [81]. Such experiments are no longer confined to preclinical model systems, and numerous early phase clinical trials started to explore these promising avenues in recurrent/refractory MBs, although therapeutic approaches rarely reflect the existing MB molecular classifications (Table 2).
Refractory, recurrent, and progressive MBs
Metastases and recurrence are the major barriers to therapeutic success, and disease recurrence is responsible for 95% of MB-associated deaths [89]. Patients who relapse after receiving upfront radiotherapy rarely survive (salvage rate < 10%) despite the multitude of treatment options [90, 91]. Most SHH tumors recur in the tumor bed, suggesting that focusing therapies at the posterior fossa could be most profitable. By contrast, Group 3 and Group 4 MBs develop distant metastases irrespectively of the type of therapy administered at diagnosis [92]. Interestingly, Group 4 patients recur later compared to the other subtypes and survive longer after recurrence. Although metastases are rare in WNT-activated MBs, recurrence may occur locally with or without systemic dissemination [91, 92].
Molecular subgroup affiliation remains stable across samples collected at diagnosis and relapse [92, 93]. However, substantial genetic divergence occurs [94–96]. About two-thirds of relapses acquire high-risk features, including TP53-MYC gene family interactions. The presence of TP53-MYC interactions is associated with rapid disease progression [95]. Whole-genome sequencing identified significant increase in somatic mutational burden post-therapy: mutations of TP53 and other genes of the TP53 pathway, such as DYNC1H1 and losses of chr14q, occurred frequently, predominantly in recurrences of SHH-MBs [94]. Putative drug targets present at diagnosis were retained in only 44% of patients post-therapy, providing an explanation why most targeted therapies fail in MB [94].
The difficulty to manage recurrent/refractory/progressive MBs is reflected in the number of clinical trials dedicated to this population: about 80% of currently recruiting studies focuses on these heavily pretreated patients, although two-thirds of the identified trials are still in early phase (Table 2).
Targeted therapies of CNS tumors irrespective of molecular subgrouping
Several trials are not explicit to MBs and recruit patients with a range of advanced solid tumors including various neoplasm of the CNS. Such is the early phase I NCT03893487 trial exploring the ability of fimepinostat (CUDC-907), a small molecule inhibitor of histone deacetylase and PI3 kinase, to penetrate the blood brain barrier (BBB) of children and young adults with various CNS tumors, including recurrent MBs of any molecular subtypes.
The efficacy of cyclin-dependent kinase (CDK) inhibitors in treating hormone receptor positive, HER2-negative advanced breast cancer boosted the interest toward their further use in other tissue types. There are currently 103 active and recruiting clinical trials as listed at clinicaltrial.gov evaluating palbociclib alone in various tissue types including primary CNS tumors. The maximum tolerated dose of palbociclib (CDK4/6 inhibitor) combined with chemotherapy (irinotecan and temozolomide) is evaluated in the NCT0370968 phase I trial in children with solid and CNS tumors. A combined ribociclib (cyclin D1/CDK4 and CDK6 inhibitor) and everolimus treatment is utilized in the NCT03387020 phase I trial in children with recurrent/refractory primary CNS tumors, including MBs, with an intact RB1 protein.
Entrectinib (RXDX-101), a TRKA/B/C, ROS1, and ALK inhibitor, is being studied in a phase I/II trial (NCT02650401) recruiting children and young adults with recurrent/refractory CNS tumors harboring NTRK1/2/3, ROS1, and ALK molecular alterations, as confirmed by a CLIA-approved lab. A National Cancer Institute (NCI) sponsored phase I trial experiments with savolitinib, a small molecule inhibitor of c-Met (NCT03598244) in primary brain tumors including recurrent/refractory MBs with MET pathway activations, defined as MET mutations/amplifications/fusions, HGF amplifications, and gain of chromosome 7. Another NCI sponsored phase I/II study (NCT02095132) evaluates the activity of adavosertib (MK-1775), a small molecule inhibitor of the tyrosine kinase WEE1, combined with irinotecan hydrochloride in relapsed or refractory solid malignancies including CNS tumors. The study also aims to evaluate predictive biomarkers of adavosertib sensitivity, such as the expression of MYC, MYCN, EZH2, H2AX, and phosphorylated WEE1. The NCT03936465 trial evaluates the safety and dosing of the investigational drug BMS-986158, a bromodomain and BET inhibitor in pediatric solid tumors with molecular features predicted to increase sensitivity to BMS-986158, consisting of MYC and MYCN translocations and amplifications and BRD4 or BRD3 translocations. Additional ongoing early phase trials evaluating the safety and tolerability of novel treatment strategies are listed in Table 2.
Targeted therapies irrespective of the tumor or tissue type
There is an increasing tendency in oncology to explore the efficacy and safety of molecularly targeted agents for sample-specific genetic alterations, irrespectively of the tumor or tissue type (Table 2). Such is the NCI-COG Pediatric MATCH (Molecular Analysis for Therapy Choice) trial (NCT03155620) that evaluates experimental treatments targeting selected molecular alterations in children and adolescents with advanced solid tumors. Drug selection is based on the results of molecular screening with genome sequencing, with at least 20 patients in each treatment arm. Six phase II clinical trials concerning recurrent/refractory MBs are currently recruiting within the program: patients harboring mutations in the FGFR1/2/3/4 pathway may receive erdafitinib (JNJ-42756493), an oral pan-FGFR inhibitor (NCT03210714); MBs with TSC loss of function mutations or PI3K/mTOR activating mutations are treated with samotolisib (Ly3023414), a PI3K/mTOR inhibitor (NCT03213678); actionable NTRK fusions are treated with larotrectinib (LOXO-101) (NCT03213704); mutations in RB or in cell cycle genes are targeted by palbociclib (NCT03526250); tumors with defects in DNA damage repair genes are targeted by olaparib (NCT03233204); and EZH2, SMARCB1, or SMARCA4 gene mutations are treated with tazemetostat, a small molecule EZH2 inhibitor.
Immunotherapies for advanced disease
The expanding number of new trials investigating immunotherapies reflects recent tendencies in oncology. Currently, the number of phase I studies utilizing an immunotherapy-based and a targeted approach is equivalent (Table 2).
Immune checkpoint inhibitors (ICIs)
Cancer cells upregulate immune checkpoints to escape T-cell-mediated immune response [97]. Groundbreaking application of immune checkpoint inhibitors in numerous solid tumors, including metastatic melanoma and renal cancer [98, 99], generated interest in their applicability in CNS cancers. The approach is especially attractive for patients with advanced disease and limited options for therapy. The success of using ICIs is limited so far – of note, most studies are retrospective and observational, with a limited sample size and unselected patient population. The anti-PD-1 agent pembrolizumab did not show clinical or histologic efficacy in pretreated patients with progressive brain tumors [100], and PD-1 inhibition with nivolumab provided limited benefits for adult patients with recurrent high-grade gliomas [101]. A retrospective review identified survival advantage of nivolumab among pediatric patients with recurrent/refractory brain tumors, especially among PD-L1-positive patients with elevated tumor mutation burden [102]. A phase I clinical trial reported the safety of ipilimumab in pediatric patients with advanced solid tumors, mostly melanomas, with increased overall survival among those with immune-related toxicities [103].
Currently, two clinical trials are investigating blockade of classical inhibitory checkpoint pathways in MBs (Table 2). The goal of the NCT02359565 phase I trial is to assess the safety and efficacy of pembrolizumab in children and young adults with recurrent/refractory/progressive brain tumors including MBs. NCT03173950, a phase II trial, evaluates the effects of biweekly nivolumab administration on objective response rate and progression-free survival of adult patients with rare CNS tumors including MBs.
The B7 homolog 3 (B7-H3), also known as CD276, is also an important checkpoint molecule with an inhibitory function on T-cell activation [104]. The NCT00089245 phase I study evaluates the effectiveness of intrathecal radioimmunotherapy using radioactive iodine labeled antibody I-8H9 against B7-H3, which, after internalization, induces cell death.
In addition to CTLA4, PD-1, and PD-L1 inhibitors, the next generation of immune-checkpoint inhibitors is focusing on other immune pathways. Molecules of the co-stimulatory pathways augment the immunological response against tumor cells. Such is the surface receptor CD40, a TNF receptor family member expressed by APCs and B cells, that stimulates cytokine expression and activation of T cells and induces tumor cell death [105]. CD40 has also direct cytotoxic effects on CD40-positive tumor cells. APX005M, a humanized IgG1κ monoclonal antibody agonist of CD40 is being evaluated in a phase I pediatric trial (NCT03389802) in patients with recurrent/refractory primary malignant CNS tumors.
Indoleamine 2,3-dioxygenase (IDO) is a tryptophan-degrading enzyme, and the produced active metabolites increase T-reg activity while decrease the activity of CD8 T cells, leading to an immunosuppressed tumor microenvironment and enhanced immune escape. IDO is frequently overexpressed in various tumor types [106] and is also associated with poor outcome and resistance to chemotherapy [107]. Indoximod, an inhibitor of the IDO pathway, is being studied with concomitant use of temozolomide in a phase I pediatric trial (NCT02502708) with an estimated completion date in December 2019. Additionally, in an NCI-funded phase II trial (NCT04049669), indoximod complemented with oral temozolomide is given to children and young adults both eligible and not-eligible for partial re-irradiation with progressive brain cancers.
Oncolytic viruses
Cancer therapy utilizing oncolytic viruses (OVs) has several advantages over traditional approaches: OVs replicate selectively only in cancerous but not in normal cells, and after infecting a few cells, OVs quickly spread through the entire tumor. The antitumor response is based on a dual mechanism of action: first, OVs induce direct lysis of tumor cells, and second, as a result, the release of neoantigens to the tumor microenvironment triggers activation of the immune system, facilitating tumor eradication [108]. Based on promising results from preclinical studies, several types of viruses are being investigated in the treatment of pediatric brain tumors [109].
A single injection of a genetically engineered herpes simplex virus (HSV) variant, rRp450 to orthotopic mouse xenografts representing Group 3 and 4 MB cells, prolonged survival and had the ability to bioactivate the prodrug cyclophosphamide within the tumor microenvironment [110]. Another set of engineered variants of the HSV, G207, and M002 displayed antitumor activity and prolonged survival of mice bearing xenografts originating from the most aggressive Group 3 or 4 pediatric MB subgroups [111]. The safety of the G207 HSV-variant augmented with radiation has already been determined in a phase I trials in adult patients with recurrent high-grade gliomas [112]. The primary HSV entry receptor nectin-1 (CD111), an adhesion molecule, is expressed in significantly higher amounts in pediatric tumor cells compared to adults and displays sensitivity to the virus in vitro, requiring a substantially lower dose [113]. The results suggest that HSV treatment could be sufficiently effective for treating pediatric brain cancers. Accordingly, a currently recruiting phase I trial (NCT03911388) evaluates the effects of engineered HSV G207 alone or in combination with a single low-dose radiation in children with recurrent/refractory brain tumors, including recurrent MBs.
Measles virus (MV) is a negative strand RNA virus that induces the fusion of neighboring cells leading to the development of giant, metabolically active, multinucleated aggregates, called syncytia, which contribute to cell death [114]. An MV that expresses thyroidal sodium iodide symporter (NIS) has been engineered to monitor viral replication – the expression of NIS also enhanced therapeutic efficacy [114]. There is currently a phase I study (NCT02962167) that aims to evaluate safety and dosing of MV-NIS for the treatment of children and young adults with recurrent MBs or recurrent atypical teratoid rhabdoid tumors (ATRT). For locally recurrent disease, the virus is introduced directly to the tumor bed. For disseminated MB, MV-NIS is injected to the subarachnoid space through lumbar puncture.
Targeted elimination may also be achieved through the highly attenuated polio/rhinovirus recombinant PVSRIPO that recognizes the poliovirus receptor CD155 widely expressed by both cancerous cells and components of the tumor microenvironment [115]. A phase I dose-finding and toxicity study on adults with recurrent supratentorial WHO grade IV malignant gliomas treated by intratumoral delivery of the recombinant polio/rhinovirus chimera identified better survival outcome compared to historical controls [116]. Following a similar approach, a current phase Ib pediatric study (NCT03043391) investigates the safety and dosing of PVSRIPO by convection-enhanced delivery (CED) using an intracerebral catheter in WHO grade III, grade IV gliomas, and other brain tumors including recurrent MBs.
Glioblastomas are lethal brain tumors with no specific therapy. Cytomegalovirus (CMV) antigens are almost universally present and homogeneously expressed in glioblastomas compared to normal brain tissues. Thus, CMV presence provides an excellent opportunity to subvert these immunogenic viral proteins to be tumor-specific targets [117]. A cost effective, two-component CMV-specific multi-epitope peptide vaccine (PEP-CMV) has been created including a synthetic long peptide of 26 amino acid residues from human pp65 and a neutralizing antibody epitope from human CMV glycoprotein B. The phase I PRiME clinical trial (NCT03299309) evaluates the safety of PEP-CMV in pediatric and young adult patients with recurrent MBs and malignant gliomas. Vaccination is preceded by temozolomide treatment to augment immunogenicity, and patients older than 18 years of age receive an intradermal tetanus vaccine booster to precondition the immune system.
CAR-T therapy
In the CAR-T therapy, genetically engineered T-cell expressing artificial chimeric antigen receptors (CAR) with both antigen-binding and T-cell activating properties are utilized. CAR-T therapy resulted in breakthroughs in treating hematological malignancies, but solid tumors pose a wide array of challenges, such as finding tumor-associated antigens enriched in tumors but not expressed in normal tissues [118]. HER2 is frequently overexpressed in MBs, and intraventricular administration of autologous HER2-CAR T cells effectively cleared orthotopically implanted MBs in the posterior fossa originating from Daoy and D283 tumor cells in a preclinical mouse MB model [119]. In the BrainChild-01 phase I trial (NCT03500991) of Seattle Children’s Hospital, autologous CD4+ and CD8+ T cells lentivirally transduced to express HER2-specific chimeric antigen receptor and EGFRt (a truncated form of the human epidermal growth factor receptor) are investigated. Engineered T cells are delivered to the tumor resection cavity or the ventricular system of recurrent/refractory MB patients with HER2-positive disease. By this delivery method, T cells do not need to penetrate the blood-brain barrier, and this can presumably provide higher efficiency compared to intravenous infusion. In another Seattle Children’s Hospital phase I trial (NCT03638167), transduced CAR-T cells expressing the EGFR806 specific-CAR and EGFRt are infused into pediatric and young adult patients with histologically diagnosed EGFR positive recurrent/refractory CNS tumors.
Natural killer cells
Natural killer (NK) cells play a major role in the immune defense against malignant disease by recognizing a broad spectrum of tumor cells without specific antigen identification [120]. NK cells are controlled by both activating and inhibitory receptors, and MB cell lines express specific ligands that are able to trigger NK activation, enabling the lysis of MB cell lines in vitro regardless of the presence or absence of the stem cell marker CD133 [121]. However, complete elimination is unlike due to inhibitory signals generated by the tumor cells. Several studies investigate ex vivo propagated, transduced, or otherwise modified NK cells, such as cells expressing dominant-negative TGF-β receptor II (DNRII) [122]. In a currently recruiting phase I clinical trial by the MD Anderson Cancer Center (NCT02271711), ex vivo propagated autologous NK cells with artificial antigen-presenting cells (aAPC) are being administered directly to the brain of pediatric patients with recurrent/refractory MBs.
Conclusions
Recent advances in MB research have expanded our understanding of MB pathogenesis and expanded the list of potential biomarkers with prognostic significance. Prospective trials are needed to confirm the validity of molecularly grounded stratifications, eventually offering more precise treatment strategies. The ongoing transition toward molecularly guided clinical interventions is slow, especially for newly diagnosed MB patients. Clinical trials aim to fine-tune invasive radio- and chemotherapies to reduce intervention-related morbidity in low- or standard-risk patients, with a particular focus on young children and rare MBs affecting adults. Studies recruiting newly diagnosed patients exploit molecular markers to some extent, especially to distinguish WNT-activated MBs utilizing mandatory testing for CTNNB1 mutational status or IHC positivity. Evaluation of the presence of MYC or MYCN amplifications to identify high-risk patients also became standard procedure. Nevertheless, incorporation of a full molecular analysis for newly diagnosed patients utilizing both the clinical risk and the tumor’s molecular subgroup allocation for therapy selection is limited to a single trial (NCT01878617).
It is especially vital to unravel novel, druggable pathways for subgroups with frequent metastases and low rates of recurrent molecular alterations, such as Group 3 and Group 4 MBs. For high-risk patients, clinical trials evaluate the effects of therapy intensification and novel chemotherapy drugs. Emergence of innovative concepts, such as integration of proteomic approaches into existing models of tumorigenesis, may expand translational opportunities [123].
The proportion of targeted and immunotherapies in recurrent/refractory MBs is increasing compared to cytotoxic strategies. Several trials consider molecular subgrouping, and novel targeted therapies are being explored irrespectively of the tumor or tissue type. Immune therapies utilize a wide range of treatment options, from immune checkpoint inhibitors, CAR-T therapies, and NK-cells to oncolytic viruses. While most trials are in an early phase, there is hope for therapeutic breakthroughs in historically difficult to treat recurrent/refractory MBs in the next decade.
Acknowledgements
Open access funding provided by Semmelweis University (SE).
Funding information
The study was supported by the KH-129581, 2018–1.3.1-VKE-2018-00032 and the 2018-2.1.17-TET-KR-00001 grants of the National Research, Development and Innovation Office, Hungary.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Key messages
• Contemporary treatment strategies fail to consider heterogeneity among medulloblastoma (MB) patients.
• Arising subgroup-specific markers of prognosis lead to a layered risk stratification of MBs, but prospective trials are needed to confirm their utility.
• Most studies on newly diagnosed patients focus on fine-tuning the intensity of current radio- and chemotherapies.
• More targeted and immunotherapy-based treatment strategies are available in recurrent and refractory MBs.
• The transition toward molecularly guided interventions for newly diagnosed MBs remains slow.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. Journal of Clinical Neuroscience. 2012;19(11):1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, Witt O, von Hoff K, Pietsch T, Northcott PA, Gajjar A, Robinson GW, Padovani L, André N, Massimino M, Pizer B, Packer R, Rutkowski S, Pfister SM, Taylor MD, Pomeroy SL. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathologica. 2016;131(6):821–831. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salloum R, Chen Y, Yasui Y, Packer R, Leisenring W, Wells E, King A, Howell R, Gibson TM, Krull KR, Robison LL, Oeffinger KC, Fouladi M, Armstrong GT. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: A report from the childhood cancer survivor study. Journal of Clinical Oncology. 2019;37(9):731–740. doi: 10.1200/jco.18.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nature Reviews. Clinical Oncology. 2011;8(9):540–549. doi: 10.1038/nrclinonc.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ning MS, Perkins SM, Dewees T, Shinohara ET. Evidence of high mortality in long term survivors of childhood medulloblastoma. Journal of Neuro-Oncology. 2015;122(2):321–327. doi: 10.1007/s11060-014-1712-y. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathologica. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathologica. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, Warnatz HJ, Sidiropoulos N, Phillips AH, Schumacher S, Kleinheinz K, Waszak SM, Erkek S, Jones DTW, Worst BC, Kool M, Zapatka M, Jäger N, Chavez L, Hutter B, Bieg M, Paramasivam N, Heinold M, Gu Z, Ishaque N, Jäger-Schmidt C, Imbusch CD, Jugold A, Hübschmann D, Risch T, Amstislavskiy V, Gonzalez FGR, Weber UD, Wolf S, Robinson GW, Zhou X, Wu G, Finkelstein D, Liu Y, Cavalli FMG, Luu B, Ramaswamy V, Wu X, Koster J, Ryzhova M, Cho YJ, Pomeroy SL, Herold-Mende C, Schuhmann M, Ebinger M, Liau LM, Mora J, McLendon R, Jabado N, Kumabe T, Chuah E, Ma Y, Moore RA, Mungall AJ, Mungall KL, Thiessen N, Tse K, Wong T, Jones SJM, Witt O, Milde T, von Deimling A, Capper D, Korshunov A, Yaspo ML, Kriwacki R, Gajjar A, Zhang J, Beroukhim R, Fraenkel E, Korbel JO, Brors B, Schlesner M, Eils R, Marra MA, Pfister SM, Taylor MD, Lichter P. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomics: The end of the beginning. Nature Reviews. Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nature Reviews. Clinical Oncology. 2014;11(12):714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 11.Shih DJ, Northcott PA, Remke M, Korshunov A, Ramaswamy V, Kool M, Luu B, Yao Y, Wang X, Dubuc AM, Garzia L, Peacock J, Mack SC, Wu X, Rolider A, Morrissy AS, Cavalli FM, Jones DT, Zitterbart K, Faria CC, Schüller U, Kren L, Kumabe T, Tominaga T, Shin Ra Y, Garami M, Hauser P, Chan JA, Robinson S, Bognár L, Klekner A, Saad AG, Liau LM, Albrecht S, Fontebasso A, Cinalli G, de Antonellis P, Zollo M, Cooper MK, Thompson RC, Bailey S, Lindsey JC, di Rocco C, Massimi L, Michiels EM, Scherer SW, Phillips JJ, Gupta N, Fan X, Muraszko KM, Vibhakar R, Eberhart CG, Fouladi M, Lach B, Jung S, Wechsler-Reya RJ, Fèvre-Montange M, Jouvet A, Jabado N, Pollack IF, Weiss WA, Lee JY, Cho BK, Kim SK, Wang KC, Leonard JR, Rubin JB, de Torres C, Lavarino C, Mora J, Cho YJ, Tabori U, Olson JM, Gajjar A, Packer RJ, Rutkowski S, Pomeroy SL, French PJ, Kloosterhof NK, Kros JM, van Meir E, Clifford SC, Bourdeaut F, Delattre O, Doz FF, Hawkins CE, Malkin D, Grajkowska WA, Perek-Polnik M, Bouffet E, Rutka JT, Pfister SM, Taylor MD. Cytogenetic prognostication within medulloblastoma subgroups. Journal of Clinical Oncology. 2014;32(9):886–896. doi: 10.1200/jco.2013.50.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. Journal of Clinical Oncology. 2011;29(11):1408–1414. doi: 10.1200/jco.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. The Lancet Oncology. 2016;17(4):484–495. doi: 10.1016/s1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson EM, Bramall A, Herndon JE, 2nd, Taylor MD, Ramaswamy V. The clinical importance of medulloblastoma extent of resection: A systematic review. Journal of Neuro-Oncology. 2018;139(3):523–539. doi: 10.1007/s11060-018-2906-5. [DOI] [PubMed] [Google Scholar]
- 15.Wolden SL, Dunkel IJ, Souweidane MM, Happersett L, Khakoo Y, Schupak K, Lyden D, Leibel SA. Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. Journal of Clinical Oncology. 2003;21(16):3079–3083. doi: 10.1200/jco.2003.11.140. [DOI] [PubMed] [Google Scholar]
- 16.Schwalbe, E. C., Lindsey, J. C., Nakjang, S., Crosier, S., Smith, A. J., Hicks, D., et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. The Lancet Oncology, 18(7), 958–971. 10.1016/S1470-2045(17)30243-7. [DOI] [PMC free article] [PubMed]
- 17.Chin AL, Moding EJ, Donaldson SS, Gibbs IC, Soltys SG, Hiniker SM, Pollom EL. Survival impact of postoperative radiotherapy timing in pediatric and adolescent medulloblastoma. Neuro-Oncology. 2018;20(8):1133–1141. doi: 10.1093/neuonc/noy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. Journal of Clinical Oncology. 1999;17(7):2127–2136. doi: 10.1200/jco.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 19.Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, Ashley DM, Sexton M, Kellie SJ, Ahern V, Gajjar A. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. International Journal of Radiation Oncology, Biology, Physics. 2008;70(3):782–787. doi: 10.1016/j.ijrobp.2007.07.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahba HA, Abu-Hegazy M, Wasel Y, Ismail EI, Zidan AS. Adjuvant chemotherapy after reduced craniospinal irradiation dose in children with average-risk medulloblastoma: A 5-year follow-up study. Journal of BUON. 2013;18(2):425–429. [PubMed] [Google Scholar]
- 21.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. The Lancet Oncology. 2006;7(10):813–820. doi: 10.1016/s1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 22.Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children's Oncology Group trial A9961. Neuro-Oncology. 2013;15(1):97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children's Cancer Group study. Journal of Clinical Oncology. 2001;19(15):3470–3476. doi: 10.1200/jco.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 24.Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, et al. Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. Journal of Clinical Oncology. 2000;18(16):3004–3011. doi: 10.1200/jco.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- 25.Michalski J, Vezina G, Burger P, Gajjar A, Pollack I, Merchant T, et al. MB-109: Preliminary results of COG ACNS0331: A phase III trial of involved field radiotherapy (IFRT) and low dose craniospinal irradiation (LD-CSI) with chemotherapy in average risk medulloblastoma: a report from the children's oncology group. Neuro-Oncology. 2016;18(Suppl 3):iii122. doi: 10.1093/neuonc/now076.104. [DOI] [Google Scholar]
- 26.Kann BH, Park HS, Lester-Coll NH, Yeboa DN, Benitez V, Khan AJ, Bindra RS, Marks AM, Roberts KB. Postoperative radiotherapy patterns of care and survival implications for medulloblastoma in young children. JAMA Oncology. 2016;2(12):1574–1581. doi: 10.1001/jamaoncol.2016.2547. [DOI] [PubMed] [Google Scholar]
- 27.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, Jones RM, MacDonald S, Pulsifer MB, Lavally B, Abrams AN, Huang MS, Marcus KJ, Tarbell NJ. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. The Lancet Oncology. 2016;17(3):287–298. doi: 10.1016/s1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 28.Moeller BJ, Chintagumpala M, Philip JJ, Grosshans DR, McAleer MF, Woo SY, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiation Oncology. 2011;6:58. doi: 10.1186/1748-717x-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. International Journal of Radiation Oncology, Biology, Physics. 2004;58(3):727–734. doi: 10.1016/s0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 30.Kahalley LS, Ris MD, Grosshans DR, Okcu MF, Paulino AC, Chintagumpala M, Moore BD, Guffey D, Minard CG, Stancel HH, Mahajan A. Comparing intelligence quotient change after treatment with proton versus photon radiation therapy for pediatric brain tumors. Journal of Clinical Oncology. 2016;34(10):1043–1049. doi: 10.1200/jco.2015.62.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoff KV, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, Benesch M, Warmuth-Metz M, Soerensen N, Zwiener I, Goette H, Schlegel PG, Pietsch T, Kortmann RD, Kuehl J, Rutkowski S. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. European Journal of Cancer. 2009;45(7):1209–1217. doi: 10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Kortmann RD, Kuhl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: Results of the German prospective randomized trial HIT '91. International Journal of Radiation Oncology, Biology, Physics. 2000;46(2):269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 33.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. Journal of Clinical Oncology. 2006;24(25):4202–4208. doi: 10.1200/jco.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 34.Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: Present concepts of stratification into risk groups. Pediatric Neurosurgery. 2003;39(2):60–67. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 35.Jakacki RI, Burger PC, Zhou T, Holmes EJ, Kocak M, Onar A, Goldwein J, Mehta M, Packer RJ, Tarbell N, Fitz C, Vezina G, Hilden J, Pollack IF. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: A Children's Oncology Group Phase I/II study. Journal of Clinical Oncology. 2012;30(21):2648–2653. doi: 10.1200/jco.2011.40.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, Kim JH, Suh YL, Joung YS, Shin HJ. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro-Oncology. 2013;15(3):352–359. doi: 10.1093/neuonc/nos304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bull KS, Spoudeas HA, Yadegarfar G, Kennedy CR. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: A follow-up study in PNET 3 trial survivors on behalf of the CCLG (formerly UKCCSG) Journal of Clinical Oncology. 2007;25(27):4239–4245. doi: 10.1200/jco.2006.08.7684. [DOI] [PubMed] [Google Scholar]
- 38.von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Muller K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. Journal of Clinical Oncology. 2016;34(34):4151–4160. doi: 10.1200/jco.2016.67.2428. [DOI] [PubMed] [Google Scholar]
- 39.Lafay-Cousin L, Smith A, Chi SN, Wells E, Madden J, Margol A, Ramaswamy V, Finlay J, Taylor MD, Dhall G, Strother D, Kieran MW, Foreman NK, Packer RJ, Bouffet E. Clinical, pathological, and molecular characterization of infant medulloblastomas treated with sequential high-dose chemotherapy. Pediatric Blood & Cancer. 2016;63(9):1527–1534. doi: 10.1002/pbc.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, et al. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children's Oncology Group. Pediatric Neurology. 2015;53(1):31–46. doi: 10.1016/j.pediatrneurol.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. The New England Journal of Medicine. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 42.Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, Doz F, Rialland X, Pichon F, Bertozzi AI, Chastagner P, Couanet D, Habrand JL, Raquin MA, le Deley MC, Kalifa C, French Society of Paediatric Oncology Treatment of medulloblastoma with postoperative chemotherapy alone: An SFOP prospective trial in young children. The Lancet Oncology. 2005;6(8):573–580. doi: 10.1016/s1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 43.Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children's Cancer Group. Journal of Clinical Oncology. 2005;23(30):7621–7631. doi: 10.1200/jco.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 44.Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U, Graf N, Berthold F, Henze G, Wolff JE, Warmuth-Metz M, Soerensen N, Emser A, Ottensmeier H, Deinlein F, Schlegel PG, Kortmann RD, Pietsch T, Kuehl J, German Pediatric Brain Tumor Study Group Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro-Oncology. 2009;11(2):201–210. doi: 10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosman R, Brown CS, DeBraganca KC, Khasraw M. Patterns of care in adult medulloblastoma: Results of an international online survey. Journal of Neuro-Oncology. 2014;120(1):125–129. doi: 10.1007/s11060-014-1525-z. [DOI] [PubMed] [Google Scholar]
- 46.Lassaletta A, Ramaswamy V. Medulloblastoma in adults: they're not just big kids. Neuro-Oncology. 2016;18(7):895–897. doi: 10.1093/neuonc/now110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. Journal of Clinical Oncology. 2006;24(12):1924–1931. doi: 10.1200/jco.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 48.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsić A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3(8):e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. Journal of Clinical Oncology. 2011;29(11):1424–1430. doi: 10.1200/jco.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, Binder ZA, Nikolsky Y, Hartigan J, Smith DR, Gerhard DS, Fults DW, VandenBerg S, Berger MS, Marie SK, Shinjo SM, Clara C, Phillips PC, Minturn JE, Biegel JA, Judkins AR, Resnick AC, Storm PB, Curran T, He Y, Rasheed BA, Friedman HS, Keir ST, McLendon R, Northcott PA, Taylor MD, Burger PC, Riggins GJ, Karchin R, Parmigiani G, Bigner DD, Yan H, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331(6016):435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramaswamy V, Remke M, Adamski J, Bartels U, Tabori U, Wang X, Huang A, Hawkins C, Mabbott D, Laperriere N, Taylor MD, Bouffet E. Medulloblastoma subgroup-specific outcomes in irradiated children: Who are the true high-risk patients? Neuro-Oncology. 2016;18(2):291–297. doi: 10.1093/neuonc/nou357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren A, Rutkowski S, McCabe M, Collins VP, Bäcklund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathologica. 2012;123(4):473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. Journal of Clinical Oncology. 2011;29(11):1400–1407. doi: 10.1200/jco.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ. Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathologica. 2011;121(3):381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, et al. Beta-catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children's Cancer Study Group Brain Tumour Committee. Journal of Clinical Oncology. 2005;23(31):7951–7957. doi: 10.1200/jco.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 56.Clifford SC, Lannering B, Schwalbe EC, Hicks D, O'Toole K, Nicholson SL, et al. Biomarker-driven stratification of disease-risk in non-metastatic medulloblastoma: Results from the multi-center HIT-SIOP-PNET4 clinical trial. Oncotarget. 2015;6(36):38827–38839. doi: 10.18632/oncotarget.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, Benner A, von Deimling A, Scheurlen W, Perry A, Croul S, Kulozik AE, Lichter P, Taylor MD, Pfister SM, Korshunov A. Adult medulloblastoma comprises three major molecular variants. Journal of Clinical Oncology. 2011;29(19):2717–2723. doi: 10.1200/jco.2011.34.9373. [DOI] [PubMed] [Google Scholar]
- 58.Zhao F, Ohgaki H, Xu L, Giangaspero F, Li C, Li P, Yang Z, Wang B, Wang X, Wang Z, Ai L, Zhang J, Luo L, Liu P. Molecular subgroups of adult medulloblastoma: A long-term single-institution study. Neuro-Oncology. 2016;18(7):982–990. doi: 10.1093/neuonc/now050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, et al. Intertumoral heterogeneity within Medulloblastoma Subgroups. Cancer Cell. 2017;31(6):737–754.e736. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, Chalhoub N, Baker SJ, Huether R, Kriwacki R, Curley N, Thiruvenkatam R, Wang J, Wu G, Rusch M, Hong X, Becksfort J, Gupta P, Ma J, Easton J, Vadodaria B, Onar-Thomas A, Lin T, Li S, Pounds S, Paugh S, Zhao D, Kawauchi D, Roussel MF, Finkelstein D, Ellison DW, Lau CC, Bouffet E, Hassall T, Gururangan S, Cohn R, Fulton RS, Fulton LL, Dooling DJ, Ochoa K, Gajjar A, Mardis ER, Wilson RK, Downing JR, Zhang J, Gilbertson RJ. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488(7409):43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, Stütz AM, Korshunov A, Reimand J, Schumacher SE, Beroukhim R, Ellison DW, Marshall CR, Lionel AC, Mack S, Dubuc A, Yao Y, Ramaswamy V, Luu B, Rolider A, Cavalli FM, Wang X, Remke M, Wu X, Chiu RY, Chu A, Chuah E, Corbett RD, Hoad GR, Jackman SD, Li Y, Lo A, Mungall KL, Nip KM, Qian JQ, Raymond AG, Thiessen NT, Varhol RJ, Birol I, Moore RA, Mungall AJ, Holt R, Kawauchi D, Roussel MF, Kool M, Jones DT, Witt H, Fernandez-L A, Kenney AM, Wechsler-Reya RJ, Dirks P, Aviv T, Grajkowska WA, Perek-Polnik M, Haberler CC, Delattre O, Reynaud SS, Doz FF, Pernet-Fattet SS, Cho BK, Kim SK, Wang KC, Scheurlen W, Eberhart CG, Fèvre-Montange M, Jouvet A, Pollack IF, Fan X, Muraszko KM, Gillespie GY, di Rocco C, Massimi L, Michiels EM, Kloosterhof NK, French PJ, Kros JM, Olson JM, Ellenbogen RG, Zitterbart K, Kren L, Thompson RC, Cooper MK, Lach B, McLendon R, Bigner DD, Fontebasso A, Albrecht S, Jabado N, Lindsey JC, Bailey S, Gupta N, Weiss WA, Bognár L, Klekner A, van Meter T, Kumabe T, Tominaga T, Elbabaa SK, Leonard JR, Rubin JB, Liau LM, van Meir E, Fouladi M, Nakamura H, Cinalli G, Garami M, Hauser P, Saad AG, Iolascon A, Jung S, Carlotti CG, Vibhakar R, Ra YS, Robinson S, Zollo M, Faria CC, Chan JA, Levy ML, Sorensen PH, Meyerson M, Pomeroy SL, Cho YJ, Bader GD, Tabori U, Hawkins CE, Bouffet E, Scherer SW, Rutka JT, Malkin D, Clifford SC, Jones SJ, Korbel JO, Pfister SM, Marra MA, Taylor MD. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schroder M. Human DEAD-box protein 3 has multiple functions in gene regulation and cell cycle control and is a prime target for viral manipulation. Biochemical Pharmacology. 2010;79(3):297–306. doi: 10.1016/j.bcp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 64.Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nature Genetics. 2017;49(1):10–16. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJ, Martin DC, Castelo-Branco P, Baskin B, Ray PN, Bouffet E, von Bueren A, Jones DT, Northcott PA, Kool M, Sturm D, Pugh TJ, Pomeroy SL, Cho YJ, Pietsch T, Gessi M, Rutkowski S, Bognar L, Klekner A, Cho BK, Kim SK, Wang KC, Eberhart CG, Fevre-Montange M, Fouladi M, French PJ, Kros M, Grajkowska WA, Gupta N, Weiss WA, Hauser P, Jabado N, Jouvet A, Jung S, Kumabe T, Lach B, Leonard JR, Rubin JB, Liau LM, Massimi L, Pollack IF, Shin Ra Y, van Meir E, Zitterbart K, Schüller U, Hill RM, Lindsey JC, Schwalbe EC, Bailey S, Ellison DW, Hawkins C, Malkin D, Clifford SC, Korshunov A, Pfister S, Taylor MD, Tabori U. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. Journal of Clinical Oncology. 2013;31(23):2927–2935. doi: 10.1200/jco.2012.48.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trubicka J, Szperl M, Grajkowska W, Karkucinska-Wieckowska A, Tarasinska M, Falana K, et al. Identification of a novel inherited ALK variant M1199L in the WNT type of medulloblastoma. Folia Neuropathologica. 2016;54(1):23–30. doi: 10.5114/fn.2016.58912. [DOI] [PubMed] [Google Scholar]
- 67.Northcott PA, Hielscher T, Dubuc A, Mack S, Shih D, Remke M, al-Halabi H, Albrecht S, Jabado N, Eberhart CG, Grajkowska W, Weiss WA, Clifford SC, Bouffet E, Rutka JT, Korshunov A, Pfister S, Taylor MD. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathologica. 2011;122(2):231–240. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leary SE, Zhou T, Holmes E, Geyer JR, Miller DC. Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: A report from the children's oncology group. Cancer. 2011;117(14):3262–3267. doi: 10.1002/cncr.25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T, Figarella-Branger D, et al. Survival and prognostic factors of early childhood medulloblastoma: An international meta-analysis. Journal of Clinical Oncology. 2010;28(33):4961–4968. doi: 10.1200/jco.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 71.Waszak SM, Northcott PA, Buchhalter I, Robinson GW, Sutter C, Groebner S, et al. Spectrum and prevalence of genetic predisposition in medulloblastoma: A retrospective genetic study and prospective validation in a clinical trial cohort. The Lancet Oncology. 2018;19(6):785–798. doi: 10.1016/s1470-2045(18)30242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tchelebi L, Ashamalla H, Graves PR. Mutant p53 and the response to chemotherapy and radiation. Sub-Cellular Biochemistry. 2014;85:133–159. doi: 10.1007/978-94-017-9211-0_8. [DOI] [PubMed] [Google Scholar]
- 74.Smith MJ, Beetz C, Williams SG, Bhaskar SS, O'Sullivan J, Anderson B, et al. Germline mutations in SUFU cause Gorlin syndrome-associated childhood medulloblastoma and redefine the risk associated with PTCH1 mutations. Journal of Clinical Oncology. 2014;32(36):4155–4161. doi: 10.1200/jco.2014.58.2569. [DOI] [PubMed] [Google Scholar]
- 75.Thalakoti S, Geller T. Basal cell nevus syndrome or Gorlin syndrome. Handbook of Clinical Neurology. 2015;132:119–128. doi: 10.1016/b978-0-444-62702-5.00008-1. [DOI] [PubMed] [Google Scholar]
- 76.Remke M, Ramaswamy V, Peacock J, Shih DJH, Koelsche C, Northcott PA, et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathologica. 2013;126(6):917–929. doi: 10.1007/s00401-013-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menyhart O, Gyorffy B. Principles of tumorigenesis and emerging molecular drivers of SHH-activated medulloblastomas. Annals of Clinical Translational Neurology. 2019;6(5):990–1005. doi: 10.1002/acn3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwalbe EC, Williamson D, Lindsey JC, Hamilton D, Ryan SL, Megahed H, Garami M, Hauser P, Dembowska-Baginska B, Perek D, Northcott PA, Taylor MD, Taylor RE, Ellison DW, Bailey S, Clifford SC. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathologica. 2013;125(3):359–371. doi: 10.1007/s00401-012-1077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamayo P, Cho YJ, Tsherniak A, Greulich H, Ambrogio L, Schouten-van Meeteren N, et al. Predicting relapse in patients with medulloblastoma by integrating evidence from clinical and genomic features. Journal of Clinical Oncology. 2011;29(11):1415–1423. doi: 10.1200/jco.2010.28.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grotzer MA, Hogarty MD, Janss AJ, Liu X, Zhao H, Eggert A, Sutton LN, Rorke LB, Brodeur GM, Phillips PC. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clinical Cancer Research. 2001;7(8):2425–2433. [PubMed] [Google Scholar]
- 81.Menyhárt O, Giangaspero F, Győrffy B. Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. Journal of Hematology & Oncology. 2019;12(1):29. doi: 10.1186/s13045-019-0712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Remke M, Hielscher T, Korshunov A, Northcott PA, Bender S, Kool M, Westermann F, Benner A, Cin H, Ryzhova M, Sturm D, Witt H, Haag D, Toedt G, Wittmann A, Schöttler A, von Bueren A, von Deimling A, Rutkowski S, Scheurlen W, Kulozik AE, Taylor MD, Lichter P, Pfister SM. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. Journal of Clinical Oncology. 2011;29(29):3852–3861. doi: 10.1200/jco.2011.36.2798. [DOI] [PubMed] [Google Scholar]
- 83.Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, Wadhwa E, Cho YJ, Stewart CF, Gilbertson RJ. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29(4):508–522. doi: 10.1016/j.ccell.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Houschyar KS, Tapking C, Borrelli MR, Popp D, Duscher D, Maan ZN, et al. Wnt pathway in bone repair and regeneration - what do we know so far. Frontiers in Cell and Developmental Biology. 2019;6:170–170. doi: 10.3389/fcell.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, et al. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian hedgehog signaling pathway. Developmental Cell. 2006;10(2):187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso P, von Hoff D, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England Journal of Medicine. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, Chintagumpala M, Takebe N, Kaste SC, Rusch M, Allen SJ, Onar-Thomas A, Stewart CF, Fouladi M, Boyett JM, Gilbertson RJ, Curran T, Ellison DW, Gajjar A. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup Medulloblastoma: Results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. Journal of Clinical Oncology. 2015;33(24):2646–2654. doi: 10.1200/jco.2014.60.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robinson GW, Kaste SC, Chemaitilly W, Bowers DC, Laughton S, Smith A, et al. Irreversible growth plate fusions in children with medulloblastoma treated with a targeted hedgehog pathway inhibitor. Oncotarget. 2017;8(41):69295–69302. doi: 10.18632/oncotarget.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pizer BL, Clifford SC. The potential impact of tumour biology on improved clinical practice for medulloblastoma: Progress towards biologically driven clinical trials. British Journal of Neurosurgery. 2009;23(4):364–375. doi: 10.1080/02688690903121807. [DOI] [PubMed] [Google Scholar]
- 90.Gajjar A, Pizer B. Role of high-dose chemotherapy for recurrent medulloblastoma and other CNS primitive neuroectodermal tumors. Pediatric Blood & Cancer. 2010;54(4):649–651. doi: 10.1002/pbc.22378. [DOI] [PubMed] [Google Scholar]
- 91.Sabel M, Fleischhack G, Tippelt S, Gustafsson G, Doz F, Kortmann R, Massimino M, Navajas A, von Hoff K, Rutkowski S, Warmuth-Metz M, Clifford SC, Pietsch T, Pizer B, Lannering B, SIOP-E Brain Tumour Group Relapse patterns and outcome after relapse in standard risk medulloblastoma: A report from the HIT-SIOP-PNET4 study. Journal of Neuro-Oncology. 2016;129(3):515–524. doi: 10.1007/s11060-016-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, Shih DJ, Luu B, Dubuc AM, Northcott PA, Schüller U, Gururangan S, McLendon R, Bigner D, Fouladi M, Ligon KL, Pomeroy SL, Dunn S, Triscott J, Jabado N, Fontebasso A, Jones DT, Kool M, Karajannis MA, Gardner SL, Zagzag D, Nunes S, Pimentel J, Mora J, Lipp E, Walter AW, Ryzhova M, Zheludkova O, Kumirova E, Alshami J, Croul SE, Rutka JT, Hawkins C, Tabori U, Codispoti KE, Packer RJ, Pfister SM, Korshunov A, Taylor MD. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. The Lancet Oncology. 2013;14(12):1200–1207. doi: 10.1016/s1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Dubuc AM, Ramaswamy V, Mack S, Gendoo DM, Remke M, Wu X, Garzia L, Luu B, Cavalli F, Peacock J, López B, Skowron P, Zagzag D, Lyden D, Hoffman C, Cho YJ, Eberhart C, MacDonald T, Li XN, van Meter T, Northcott PA, Haibe-Kains B, Hawkins C, Rutka JT, Bouffet E, Pfister SM, Korshunov A, Taylor MD. Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathologica. 2015;129(3):449–457. doi: 10.1007/s00401-015-1389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, Remke M, Cavalli FM, Ramaswamy V, Lindsay PE, Jelveh S, Donovan LK, Wang X, Luu B, Zayne K, Li Y, Mayoh C, Thiessen N, Mercier E, Mungall KL, Ma Y, Tse K, Zeng T, Shumansky K, Roth AJ, Shah S, Farooq H, Kijima N, Holgado BL, Lee JJ, Matan-Lithwick S, Liu J, Mack SC, Manno A, Michealraj KA, Nor C, Peacock J, Qin L, Reimand J, Rolider A, Thompson YY, Wu X, Pugh T, Ally A, Bilenky M, Butterfield YS, Carlsen R, Cheng Y, Chuah E, Corbett RD, Dhalla N, He A, Lee D, Li HI, Long W, Mayo M, Plettner P, Qian JQ, Schein JE, Tam A, Wong T, Birol I, Zhao Y, Faria CC, Pimentel J, Nunes S, Shalaby T, Grotzer M, Pollack IF, Hamilton RL, Li XN, Bendel AE, Fults DW, Walter AW, Kumabe T, Tominaga T, Collins VP, Cho YJ, Hoffman C, Lyden D, Wisoff JH, Garvin JH Jr, Stearns DS, Massimi L, Schüller U, Sterba J, Zitterbart K, Puget S, Ayrault O, Dunn SE, Tirapelli DP, Carlotti CG, Wheeler H, Hallahan AR, Ingram W, MacDonald T, Olson JJ, van Meir E, Lee JY, Wang KC, Kim SK, Cho BK, Pietsch T, Fleischhack G, Tippelt S, Ra YS, Bailey S, Lindsey JC, Clifford SC, Eberhart CG, Cooper MK, Packer RJ, Massimino M, Garre ML, Bartels U, Tabori U, Hawkins CE, Dirks P, Bouffet E, Rutka JT, Wechsler-Reya RJ, Weiss WA, Collier LS, Dupuy AJ, Korshunov A, Jones DT, Kool M, Northcott PA, Pfister SM, Largaespada DA, Mungall AJ, Moore RA, Jabado N, Bader GD, Jones SJ, Malkin D, Marra MA, Taylor MD. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016;529(7586):351–357. doi: 10.1038/nature16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hill RM, Kuijper S, Lindsey JC, Petrie K, Schwalbe EC, Barker K, Boult JK, Williamson D, Ahmad Z, Hallsworth A, Ryan SL, Poon E, Robinson SP, Ruddle R, Raynaud FI, Howell L, Kwok C, Joshi A, Nicholson SL, Crosier S, Ellison DW, Wharton SB, Robson K, Michalski A, Hargrave D, Jacques TS, Pizer B, Bailey S, Swartling FJ, Weiss WA, Chesler L, Clifford SC. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27(1):72–84. doi: 10.1016/j.ccell.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, Croul S, Bouffet E, Fults DW, Eberhart CG, Garzia L, van Meter T, Zagzag D, Jabado N, Schwartzentruber J, Majewski J, Scheetz TE, Pfister SM, Korshunov A, Li XN, Scherer SW, Cho YJ, Akagi K, MacDonald T, Koster J, McCabe M, Sarver AL, Collins VP, Weiss WA, Largaespada DA, Collier LS, Taylor MD. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482(7386):529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. New England Journal of Medicine. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blumenthal DT, Yalon M, Vainer GW, Lossos A, Yust S, Tzach L, Cagnano E, Limon D, Bokstein F. Pembrolizumab: First experience with recurrent primary central nervous system (CNS) tumors. Journal of Neuro-Oncology. 2016;129(3):453–460. doi: 10.1007/s11060-016-2190-1. [DOI] [PubMed] [Google Scholar]
- 101.Kurz SC, Cabrera LP, Hastie D, Huang R, Unadkat P, Rinne M, Nayak L, Lee EQ, Reardon DA, Wen PY. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology. 2018;91(14):e1355–e1359. doi: 10.1212/wnl.0000000000006283. [DOI] [PubMed] [Google Scholar]
- 102.Gorsi HS, Malicki DM, Barsan V, Tumblin M, Yeh-Nayre L, Milburn M, et al. Nivolumab in the treatment of recurrent or refractory pediatric brain tumors: A single institutional experience. Journal of Pediatric Hematology/Oncology. 2019;41(4):e235–e241. doi: 10.1097/mph.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 103.Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, Delbrook C, Lodish M, Bishop R, Wolchok JD, Streicher H, Mackall CL. Phase I clinical trial of Ipilimumab in pediatric patients with advanced solid tumors. Clinical Cancer Research. 2016;22(6):1364–1370. doi: 10.1158/1078-0432.ccr-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: Targeting B7-H3 (CD276) for human cancer immunotherapy. Clinical Cancer Research. 2016;22(14):3425–3431. doi: 10.1158/1078-0432.ccr-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clinical Cancer Research. 2007;13(4):1083–1088. doi: 10.1158/1078-0432.ccr-06-1893. [DOI] [PubMed] [Google Scholar]
- 106.Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. Journal for Immunotherapy of Cancer. 2015;3:51. doi: 10.1186/s40425-015-0094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): Only an enzyme or a checkpoint controller? Journal of Oncological Sciences. 2017;3(2):52–56. doi: 10.1016/j.jons.2017.04.001. [DOI] [Google Scholar]
- 108.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nature Reviews. Drug Discovery. 2015;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Varela-Guruceaga M, Tejada-Solís S, García-Moure M, Fueyo J, Gomez-Manzano C, Patiño-García A, et al. Oncolytic viruses as therapeutic tools for pediatric brain tumors. Cancers. 2018;10(7):226. doi: 10.3390/cancers10070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Studebaker AW, Hutzen BJ, Pierson CR, Haworth KB, Cripe TP, Jackson EM, Leonard JR. Oncolytic herpes virus rRp450 shows efficacy in Orthotopic Xenograft group 3/4 Medulloblastomas and atypical Teratoid/Rhabdoid tumors. Mol Ther Oncolytics. 2017;6:22–30. doi: 10.1016/j.omto.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedman GK, Moore BP, Nan L, Kelly VM, Etminan T, Langford CP, Xu H, Han X, Markert JM, Beierle EA, Gillespie GY. Pediatric medulloblastoma xenografts including molecular subgroup 3 and CD133+ and CD15+ cells are sensitive to killing by oncolytic herpes simplex viruses. Neuro-Oncology. 2016;18(2):227–235. doi: 10.1093/neuonc/nov123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Markert JM, Razdan SN, Kuo HC, Cantor A, Knoll A, Karrasch M, Nabors LB, Markiewicz M, Agee BS, Coleman JM, Lakeman AD, Palmer CA, Parker JN, Whitley RJ, Weichselbaum RR, Fiveash JB, Gillespie GY. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Molecular Therapy. 2014;22(5):1048–1055. doi: 10.1038/mt.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Friedman GK, Bernstock JD, Chen D, Nan L, Moore BP, Kelly VM, Youngblood SL, Langford CP, Han X, Ring EK, Beierle EA, Gillespie GY, Markert JM. Enhanced sensitivity of patient-derived pediatric high-grade brain tumor Xenografts to Oncolytic HSV-1 Virotherapy correlates with Nectin-1 expression. Scientific Reports. 2018;8(1):13930. doi: 10.1038/s41598-018-32353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aref S, Bailey K, Fielding A. Measles to the rescue: A review of oncolytic measles virus. Viruses. 2016;8(10):294. doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gromeier M, Nair SK. Recombinant poliovirus for cancer immunotherapy. Annual Review of Medicine. 2018;69:289–299. doi: 10.1146/annurev-med-050715-104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent glioblastoma treated with recombinant poliovirus. New England Journal of Medicine. 2018;379(2):150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, 2nd, Healy P, McLendon R, Friedman AH, Friedman HS, Bigner D, Vlahovic G, Mitchell DA, Sampson JH. Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clinical Cancer Research. 2017;23(8):1898–1909. doi: 10.1158/1078-0432.ccr-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez M, Moon EK. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Frontiers in Immunology. 2019;10:128–128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, et al. Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. Journal for Immunotherapy of Cancer. 2018;6(1):30–30. doi: 10.1186/s40425-018-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perez-Martinez A, Fernandez L, Diaz MA. The therapeutic potential of natural killer cells to target medulloblastoma. Expert Review of Anticancer Therapy. 2016;16(6):573–576. doi: 10.1080/14737140.2016.1184978. [DOI] [PubMed] [Google Scholar]
- 121.Castriconi R, Dondero A, Negri F, Bellora F, Nozza P, Carnemolla B, Raso A, Moretta L, Moretta A, Bottino C. Both CD133+ and CD133- medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. European Journal of Immunology. 2007;37(11):3190–3196. doi: 10.1002/eji.200737546. [DOI] [PubMed] [Google Scholar]
- 122.Powell AB, Yadavilli S, Saunders D, Van Pelt S, Chorvinsky E, Burga RA, et al. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. Journal of Translational Medicine. 2019;17(1):321–321. doi: 10.1186/s12967-019-2055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Forget A, Martignetti L, Puget S, Calzone L, Brabetz S, Picard D, et al. Aberrant ERBB4-SRC signaling as a hallmark of group 4 medulloblastoma revealed by integrative phosphoproteomic profiling. Cancer Cell. 2018;34(3):379–395.e377. doi: 10.1016/j.ccell.2018.08.002. [DOI] [PubMed] [Google Scholar]