Abstract
Background: Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) is associated with high rates of treatment failure and poor outcome. Activation of ABL/Src family kinases is found in ~10% of Ph-like ALL, which can be therapeutically targeted by tyrosine kinase inhibitors. LYN is a member of the ABL/Src-tyrosine kinase family. Somatic LYN rearrangements are found in 5 cases of hematopoietic malignancies so far, although none of them were treated with tyrosine kinase inhibitors.
Case presentation: A 6-year-old boy with relapsed B-ALL had no response to reinduction chemotherapy. He was then treated with the ABL1 tyrosine kinase inhibitor dasatinib and achieved complete remission within 2 weeks. Haploidentical allogenic stem cell transplantation (allo-HSCT) was subsequently performed and maintenance therapy with dasatinib initiated 8 weeks post-transplantation. He has been in minimal residual disease negative remission for 10 months after allo-HSCT.
Result: His bone marrow karyotype showed a balanced translocation between chromosomes 8 and 17, leading to a NCOR1-LYN fusion gene confirmed with sequencing.
Conclusion: Although LYN overexpression is described in many AML and B-ALL patients, intragenic LYN rearrangement is a rare event. For the first time, we present evidence that dasatinib is effective in treating a pediatric B-ALL with NCOR-LYN fusion.
Keywords: NCOR1-LYN, fusion gene, dasatinib, ALL, pediatric
Background
In spite of the excellent prognosis of pediatric B-ALL, disease relapse still reaches as high as 15–20% of these patients, which remains the major cause of leukemia-related death. High-risk B-ALL, such as Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL), is associated with higher rates of treatment failure, elevated minimal residual disease (MRD) levels, early relapse, and poor outcome (1). Activation of ABL/Src family kinases or JAK family kinases resulted from chromosome rearrangements occur frequently in patients with such characters, which can be therapeutically targeted by ABL/Src or JAK inhibitors, respectively (2). LYN is a member of the ABL/Src-tyrosine kinase family. Intragenic LYN rearrangement has been reported in 5 cases of hematopoietic malignancies so far (3–8) (Table 1). Although in vitro studies showed that the ABL/Src inhibitors were capable of blocking LYN's kinase activities, their clinical efficacy in real patients remains unknown (9). Here, we report a pediatric relapsed B-ALL with a t(8;17)(q12;p11.2)/NCOR1-LYN fusion showing robust and rapid response to dasatinib monotherapy.
Table 1.
Characteristics of the reported and the present cases with a LYN rearrangement.
| Cases | Age(year)/gender | Disease | Initial WBC counts | Karyotype | Fusion gene | Additional genetic changes | Relapse | TKI | Allo-HSCT | Clinical outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Tanaka et al. (4) | 21/male | PMF* | 25.5 × 109/L | 46,XY,ins(12;8)(p13;q11q21) | ETV6-LYN | Unknown | No | Imatinib | Yes | Dead |
| Telford et al. (5) | 46/male | MPN* | 17.2 × 109/L | 46,XY,der(8)inv(q12.1q21.1)t (8;12)(q12.1;p13),der(12)t(8;12) (q12.1;p13)[2]# |
ETV6-LYN | Unknown | No | No | No | Dead |
| Ma et al. (6) | 41/male | AML | 16.1 × 109/L | 47,XY,add(1)(p13), der(12)t(1;12)(p13;p12),+mar [19]/46,XY[1] |
ETV6-LYN | No | No | No | Yes | Unknown |
| Reshmi et al. (9) | Unknown | B-ALL | Unknown | Unknown | GATAD2A-LYN | Unknown | Unknown | Unknown | Unknown | Unknown |
| Yano et al. (7) and Imamura et al. (8) | 8/female | B-ALL | 293 × 109/L | No metaphases | NCOR1-LYN | Deletion of IKZF1, BTG1,CDK N2A/2B |
Yes | No | Yes | CR* |
| The present case | 6/male | B-ALL | 883 × 109/L | 46,XY,t(8;17)(q12;p11.2[10]/48, idem,+der(17)t(9;17),+22/46,XY[1]) [9]/46, XY[9] |
NCOR1-LYN | Deletion of IKZF1, CDKN2A | Yes | Dasatinib | Yes | CMR* |
CR: complete remission; CMR: complete molecular remission; MPN: myeloproliferative neoplasm; PMF: primary myelofibrosis.
The intact karyotype was as: 46,XY,der(8)inv(q12.1q21.1)t(8;12)(q12.1;p13),der(12)t(8;12)(q12.1;p13)[2]/47,sl,+der(8)inv(8)t(8;12)[5].
/48,sdl1,+der(8)inv(8)t(8;12)[2]/46,XY[2].
Case Presentation
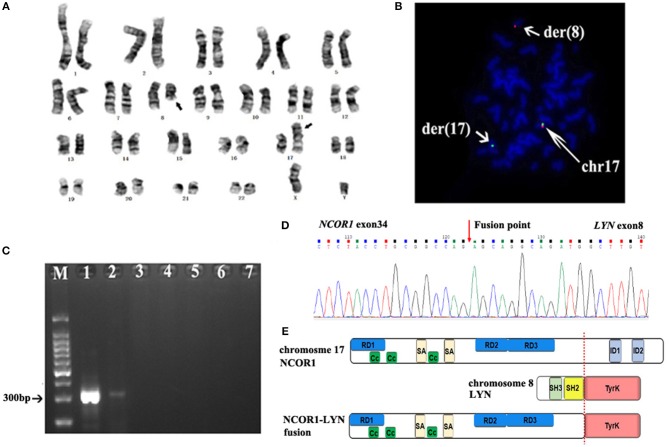
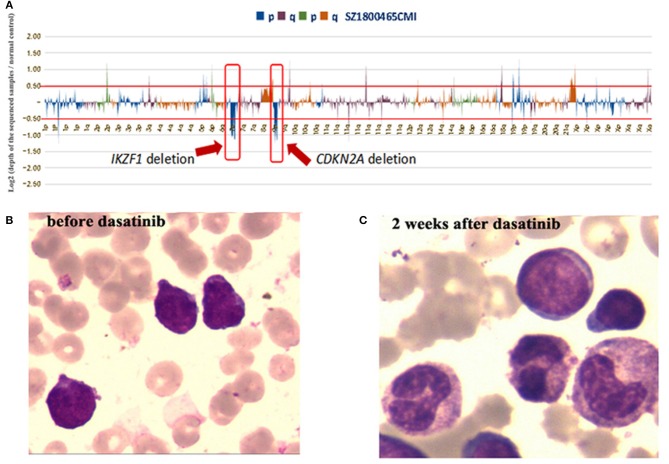
The patient presented with swollen gums in March 2015 in an outside hospital. A complete peripheral blood cell count showed leukocytes 883 × 109/L, Hb 56g/L and platelets 41 × 109/L. Bone marrow histology showed 97.6% of blasts, which were negative for myeloid peroxidase. Flow cytometry demonstrated 91.3% of blasts that were positive for CD10, CD19, CD22, and cyCD79a. Karyotype analysis of the bone marrow specimen found only 2 metaphases with normal 46, XY karyotype. Fluorescence in situ hybridization (FISH) studies were negative for BCR/ABL1, ETV6/RUNX1 translocations and KMT2A (MLL), MYC and PDGFRB rearrangements. Result of multiplex PCR covering 41 fusion genes commonly detected in ALL was negative. A diagnosis of B-ALL was established. The patient was treated with daunorubicin (DNR), vincrinstine (VCR), PEG asparaginase (PEG-ASP) according to the Chinese Children's Cancer Group (CCCG)-2015-ALL protocol (10) and achieved complete hematological remission at the end of induction chemotherapy. Minimal residual disease (MRD) based on flow cytometry remained positive (≥1 × 10−4) during the subsequent chemotherapy. HSCT was not performed due to parents' concern on potential HSCT-related complications. The patient received 3 years' chemotherapy following the CCCG-2015-ALL protocol. Consolidation chemotherapy included 4 cycles of high-dose methotrexate (MTX), followed with 5 cycles of combined chemotherapy with dexamethasone (Dex), DNR, VCR, PEG-ASP, and cytarabine. Maintenance therapy comprised cycles of 6-mercaptopurine and MTX, which was completed in March 2018. Unfortunately, disease relapsed in September 2018 (42 months after the initial diagnosis), and he was admitted to our hospital. A complete peripheral blood cell count showed leukocytes 44.8 × 109/L, Hb 64 g/L and platelets 99 × 109/L. Blast counts of bone marrow were 71.5% by histology and 84.1% by flow cytometry, blasts were positive for CD10, CD19, CD22, CD38, and cyCD79a and negative for CD20. Chromosome analysis of the bone marrow specimen showed 46,XY,t(8;17)(q12;p11.2),9qh+[10]/48,idem,+der(17)t(8;17),+22[9]/46,XY,9qh+[1] (Figure 1A). Fluorescence in situ hybridization (FISH) analysis with a panel of FISH probes specific to Ph-like B-ALL, including ABL1, ABL2, JAK2, CRLF2, and EPOR, were all negative (data not shown). FISH with a chromosome 17 centromere probe and a TP53 probe showed that one of the TP53 signals was relocated to the der (8) chromosome, consistent with a chromosome 17 breakpoint which was centromeric to TP53 (Figure 1B). Because NCOR1 is located at 17p11.2, we assumed that the t(8;17)(q12;p11.2) led to NCOR1/LYN fusion. PCR with primers specific to NCOR1 (5′ -CGTACAACTCTGCTTCCATGTCTC-3′) and LYN (5′-GCCACCTTGGTACTGTTGTTATAGTAAC-3′) showed a sharp band, with a size consistent with the NCOR1/LYN fusion; while no such band was detected using placenta control RNA template (Figure 1C). Sanger sequencing of the PCR band confirmed that the NCOR1 exon 34 was fused to LYN exon 8 (Figure 1D). In addition to the NCOR-LYN fusion, several gene mutations were also observed in a concurrent next-generation sequencing assay, ARID1A Ala41Val with a variant allele frequency (VAF) of 63.1%, KRAS Gly12Ala with VAF of 0.6%, NRAS Gln61His with VAF of 2.2%, PAX5 Arg140Leu with VAF of 39.9% and ZNF292 Asn1695del with VAF of 43.4% (data not shown), and deletion of CDKN2A and IKZF1 (Figure 2A). Unfortunately, we are unable to determine whether these genomic changes, including the NCOR-LYN fusion, are also present in the diagnostic specimen, due to lack of sample.
Figure 1.
G-banding analysis of the BM sample at relapse, which showed the balanced translocation t(8;17)(q12;p11.2). (B) FISH with a chromosome 17 centromere probe (green) and a TP53 probe (red) showed one of the TP53 signals on the der (8) chromosome, suggesting that the chromosome 17p breakpoint is centromeric to TP53 on 17p13. (C) RT-PCR showed various levels of NCOR1-LYN fusion transcript in bone marrow specimens. Lane 1: relapse sample; lane 2: 2 weeks after dasatinib; lane 3: 1 month post allo-HSCT; lane 4: 2 months post allo-HSCT; lane 5: 3 months post allo-HSCT; lane 6: 10 months post allo-HSCT; lane 7: negative control. (D) Sanger sequencing result of the NCOR1-LYN fusion gene, confirming a fusion between exon 34 of NCOR1 and exon 8 of LYN. (E) Schematic representation of the predicted domain structure of the NCOR1-LYN fusion.
Figure 2.
NGS analysis of the bone marrow sample at relapse showed deletions of CDKN2A and IKZF1. (B,C) Bone marrow smears before and after dasatinib therapy.
The patient was initially treated with mitoxantrone, vincristine and Dex. After completion of chemotherapy, bone marrow morphology still showed 61.5% of blasts (Figure 2B). The patient was then treated with dasatinib (60 mg/m2, once daily) as monotherapy. Two weeks after dasatinib treatment, bone marrow aspirates showed only 1% of blasts by histology (Figure 2C) and 0.1% of blasts by flow cytometry. Karyotype analysis of the bone marrow was normal. RT-PCR assays showed significantly decreased fusion transcript (Figure 1C). Haploidentical HSCT was performed 80 days after dasatinib therapy. No symptoms of graft vs. host disease were observed. Therefore, dasatinib was started 8 weeks post allo-HSCT for prevention of relapse. He has been tolerated with dasatinib very well and remained in MRD negative remission for 10 months now post allo-HSCT, based on both flow cytometry and RT-PCR assays (Figure 1C). Timeline of the treatment was shown in Supplementary Figure 1.
Discussion
LYN, located on 8q12.1, is highly expressed in hematopoietic cells and plays roles in B-cell signaling, mast cell degranulation and erythroid differentiation (11). Four major functional domains of LYN include Src Homology 2 (SH2), SH3, proline-rich hinge region (P), and tyrosine kinase domain. In its inactivation status, SH2 and SH3 bind to LYN's phosphorylated carboxyl terminus (Y508) and P region, respectively, to ensure an inactivation conformation. LYN is activated when Y508 is dephosphorylated or the intramolecular binding released by competitive interaction with SH2 and/or SH3 (12). NCOR1 plays important roles in cellular metabolism, cell proliferation and neural stem cell differentiation (13–15). NCOR1 interacts with nuclear hormone receptors and recruits multiple histone deacetylase enzymes including HDAC1-4 and HDAC7 to form inhibitory chromatin structures. Four major functional domains of NCOR1 include repression domains (RD), coiled-coil domains (CC), SANT-like domains (SA) (for HDAC binding) and interactive domains (ID) (for nuclear hormone receptor binding). NCOR1-LYN fusion contains the 5′NCOR1, including RD, SA, and CC domains, and the 3′ LYN with an intact protein kinase domain (Figure 1E). Because CC function as oligomerization domains for a wide variety of proteins and are capable of both homo-oligomerization and hetero-oligomerization (13), we propose an oncogenic model that NCOR1-LYN homo-dimerization, driven by the CC domains from NCOR1, leads to trans-autophosphorylation within the activation loop of LYN kinase domain and constitutive LYN signaling.
Although LYN is over-expressed in acute myeloid leukemia (AML) and Ph+-ALL, intragenic rearrangements involving LYN are rare, with only 5 cases reported so far (Table 1) (4–8). The fusion partners of LYN include GATAD2A, ETV6, and NCOR. GATAD2A-LYN was reported in a patient with B-ALL with no clinic details of the disease (9). ETV6-LYN was reported in 2 patients with MPN and 1 patient with de novo AML. All three cases were resistant to conventional chemotherapy, progressed rapidly and succumbed to diseases in a short period of time (4–6). One of these patients was treated with imatinib with no response (4), although dasatinib was not used in any of these cases. Interestingly, ETV6-LYN transfected murine Ba/F3 hematopoietic cells were sensitive to dasatinib but not imatinib (4). Yano et al. reported the first B-ALL case with a NCOR1-LYN fusion gene (7, 8). Thus, our patient here confirmed that the NCOR1-LYN fusion is a recurrent event in B-ALL. Both patients had the same breakpoints at intron 7 of LYN and intron 34 of NCOR1. IKZF1 gene defects occur in about 70% of Ph positive and Ph-like ALL cases and mediate therapy resistance (16). Therefore, we consider that IKZF1 deletion might contribute to chemoresistance of this patient. Also, deletions of IKZF1 and CDKN2A were observed in both cases. Clinically, both were pediatric B-ALL with high WBC counts at diagnosis who relapsed after chemotherapy. The previous case didn't receive tyrosine kinase inhibitors and survived after allo-HSCT (Table 1). Together these studies suggest that the pediatric B-ALL with NCOR-LYN fusion may have similar oncogenic mechanisms and clinical course.
Conclusion
In summary, we present the first case of B-ALL with NCOR1-LYN fusion who showed a quick and robust response to dasatinib. Whether or not leukemia with LYN overexpression, in the absence of LYN rearrangement, is responsive to dasatinib is probably worth further evaluation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Consent
Written informed consent was obtained from the the patients' legal guardians for publication of this case report and the accompanying images.
Author Contributions
C-XY, TC, and SX designed and interpreted data of the genetic analysis. Y-HZ and M-QZ performed flowcytometry analysis. H-PD, JY, ZL, X-MZ, D-PW, and X-WT treated the patient. H-PD and SX wrote the manuscript. D-PW and X-WT revised the manuscript. All authors approved the final version of the manuscript.
Conflict of Interest
C-XY, TC, PC, and Y-HZ are employed by the company Sano Suzhou Precision Medicine Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by research grants from the National Natural Science Foundation of China (81873443), Frontier Clinical Technical Project of the Science and Technology Department of Jiangsu Province (BE2017655), the Jiangsu Provincial Medical Talent (ZDRCA2016045), Major Natural Science Research Projects in institutions of higher education of Jiangsu Province (19KJA210002), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Society and Science Development Program (BE2016678).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00359/full#supplementary-material
Timeline of the treatment.
References
- 1.Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen IM, et al. High frequency and poor outcome of philadelphia chromosome-Like acute lymphoblastic leukemia in adults. J Clin Oncol. (2017) 35:394–401. 10.1200/JCO.2016.69.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts KG, Yang YL, Payne-Turner D, Lin W, Files JK, Dickerson K, et al. Oncogenic role and therapeutic targeting of ABL-class and JAK-STAT activating kinase alterations in Ph-like ALL. Blood Adv. (2017) 1:1657–71. 10.1182/bloodadvances.2017011296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka H, Takeuchi M, Takeda Y, Sakai S, Abe D, Ohwada C, et al. Identification of a novel TEL-Lyn fusion gene in primary myelofibrosis. Leukemia. (2010) 24:197–200. 10.1038/leu.2009.167 [DOI] [PubMed] [Google Scholar]
- 4.Telford N, Alexander S, McGinn OJ, Williams M, Wood KM, Bloor A, et al. Myeloproliferative neoplasm with eosinophilia and T-lymphoblastic lymphoma with ETV6-LYN gene fusion. Blood Cancer J. (2016) 6:e412. 10.1038/bcj.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma ESK, Wan TSK, Au CH, Ho DN, Ma SY, Ng MHL, et al. Next-generation sequencing and molecular cytogenetic characterization of ETV6-LYN fusion due to chromosomes 1, 8 and 12 rearrangement in acute myeloid leukemia. Cancer Genet. (2017) 218–219:15–9. 10.1016/j.cancergen.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Yano M, Imamura T, Asai D, Kiyokawa N, Nakabayashi K, Matsumoto K, et al. Identification of novel kinase fusion transcripts in paediatric B cell precursor acute lymphoblastic leukaemia with IKZF1 deletion. Br J Haematol. (2015) 171:813–7. 10.1111/bjh.13757 [DOI] [PubMed] [Google Scholar]
- 7.Imamura T, Kiyokawa N, Kato M, Imai C, Okamoto Y, Yano M, et al. Characterization of pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia with kinase fusions in Japan. Blood Cancer J. (2016) 6:e419. 10.1038/bcj.2016.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children's Oncology Group. Blood. (2017) 129:3352–61. 10.1182/blood-2016-12-758979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts KG. Why and how to treat Ph-like ALL? Best Pract Res Clin Haematol. (2018) 31:351–6. 10.1016/j.beha.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Yu J, Zhu X, Hu S, Zhu Y, Jiang H, et al. Chinese Children's Cancer Group childhood acute lymphoblastic leukaemia (ALL) 2015 study group (CCCG-ALL-2015). Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children's Cancer Group. Arch Dis Child. (2019) 6:522–9. 10.1136/archdischild-2018-316181 [DOI] [PubMed] [Google Scholar]
- 11.Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun Signal. (2012) 10:21. 10.1186/1478-811X-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. (2005) 22:9–18. 10.1016/S1074-7613(04)00381-4 [DOI] [PubMed] [Google Scholar]
- 13.Wong MM, Guo C, Zhang J. Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. Am J Clin Exp Urol. (2014) 2:169–87. [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. (2013) 52:769–82. 10.1016/j.molcel.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. (2002) 419:934–9. 10.1038/nature01156 [DOI] [PubMed] [Google Scholar]
- 16.Marke R, van Leeuwen FN, Scheijen B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica. (2018) 103:565–74. 10.3324/haematol.2017.185603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timeline of the treatment.