Table 3.
Representative four phenotypes and one normal aging characteristic with its progression between two visits.
| Phenotype definition (and prevalence) | Declined NPT tasks & volume loss on brain regions | Impaired cognitive areas | Amyloid beta (pg/ml) | Tau (pg/ml) | P-Tau (pg/ml) | CDR-SB |
|---|---|---|---|---|---|---|
|
Phenotype 1 Memory decline (40.3%, n = 345) |
Word recall; Writing a check; Paying bills, or balancing checkbook; Lh caudal anterior cingulate | 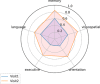 |
816.6865.3 | 327.2 310.9 | 32.4 30.3 | 2.35.5* |
|
Phenotype 2 Language deficit (18.9%, n = 162) |
Comprehension; Orientation; Word Finding; Spoken language | 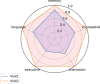 |
709.3 837.2 * | 355.1 314.8* | 35.1 30.6* | 4.27* |
|
Phenotype 28 Progressed AD (36.8%, n = 315) |
Writing checks, paying bills, or balancing checkbook; Recall instructions |
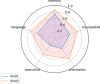 |
796.3 868.1* | 332.4 316.7 | 32.9 30.9 | 3.15.8* |
|
Phenotype 21 Visuospatial planning dysfunction (34.9%, n = 299) |
Number cancellation; Ideational Praxis; Naming |
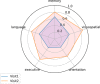 |
83 4.1862.5 | 32 1.3 312.2 | 31.6 30.2 | 2.35.8* |
|
Phenotype 4 Normal aging (99.9%, n = 856) |
Paying attention to and understanding TV program, book, or magazine Wm rh pericalcarine; wm rh lingual; wm lh lingual; wm rh insula; wm rh parahippocampal; left hippocampus (and other 45 areas) |
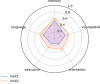 |
918.9895.4 | 301.7 304.3 | 29. 4 29.5 | 1.63.7* |
We listed the neuropsychological tests (NPT) and brain regions with highest involvement or membership on each phenotype. Prevalence is computed as the number of patients who have the characteristic of the phenotype (i.e., membership value > 10−5)/total number of patients. We plotted five cognitive areas (memory, visuospatial, orientation, executive, and language function) using ADAS-cog (i.e., Q1, Q4, and Q9 for memory; Q3 and Q6 for visuospatial; Q7 for orientation; Q2 for executive; Q8, Q10, Q11, Q12, and Q5 for language). We normalized the partial sum of cognitive scores by dividing it by maximum values. We presented the two visits’ mean values of various biomarkers (incl uding Amyloid-beta, Tau, P-Tau, and CDR-SB) to see underlying progression. Due to limited space, comprehensive variables including demographics, ApoE allele, and RAVLT for each phenotype can be found in Supplementary Table S3. We examined statistical significance on the change of biomarkers using weighted t-test, where the weights are obtained from the patient’s membership value to each phenotype.
Abbreviations: CDR-SB = Clinical dementia rating–sum of boxes; P-Tau = Phosphorylated Tau; wm = white matter; rh = right hemisphere; lh = left hemisphere; * if p-value <0.1 for weighted t-test to evaluate the values from first and second visits change significantly.
