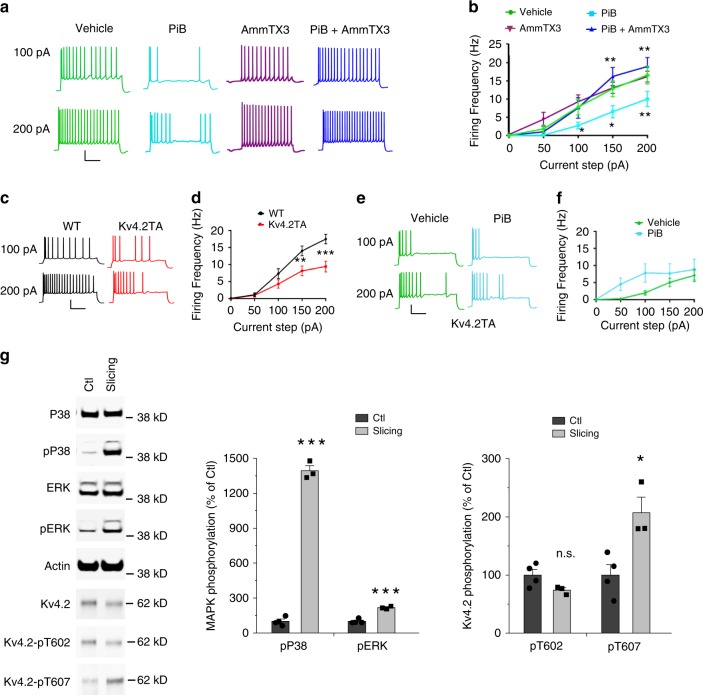
Fig. 5. P38-Pin1-Kv4.2 pathway regulates neuronal excitability.
a, b Pin1 inhibitor PiB (4 µM) reduces pyramidal cell excitability in WT mouse hippocampal brain slices. a Current steps at 100 pA and 200 pA result in reduced firing frequency with PiB application (teal) (n = 13) relative to vehicle (green) (n = 14). This reduction is rescued by co-application of AmmTX3 (250 nM) (blue) (n = 7). Scale: 30 mV / 250 ms. b, PiB significantly reduces AP firing frequency relative to vehicle in response to 100, 150, and 200 pA somatic current injections. This reduction is rescued by AmmTX3 application. Two-way ANOVA, *p < 0.05, **p < 0.01. c, d Mutation of Kv4.2 T607 Pin1 binding site phenocopies pharmacological inhibition of Pin1 in WT. c Pyramidal cells from Kv4.2TA slices display reduced firing frequency relative to WT over a range of increasing current injections. Scale: 30 mV/250 ms. d AP firing frequency is significantly reduced after 150 and 200 pA somatic current injections in Kv4.2TA (n = 22) relative to WT (n = 20), two-way ANOVA, **p < 0.01, ***p < 0.001. e, f Pin1 inhibition with PiB has no effect in Kv4.2TA mice. e Pyramidal cells from Kv4.2TA slices display similar AP firing patterns when treated with vehicle (n = 14) and PiB (n = 14). Scale: 30 mV/250 ms. f No significant changes are observed in Kv4.2TA pyramidal cell AP firing frequency with PiB exposure, Two-Way ANOVA, p > 0.05. g Brain slicing and recovery activates p38 and increases pT607 of Kv4.2. Adult mouse brains were either sliced as for electrophysiological recordings or dissected as for biochemical assays. Same brain regions were used. n = 4 for ctl and 3 for slicing. T-test. *p < 0.05, ***p < 0.001. Data are presented as mean ± SEM.