Figure 2. Dysregulated mortalin-PBD causes lethality in B-RafV600E-expressing cell.
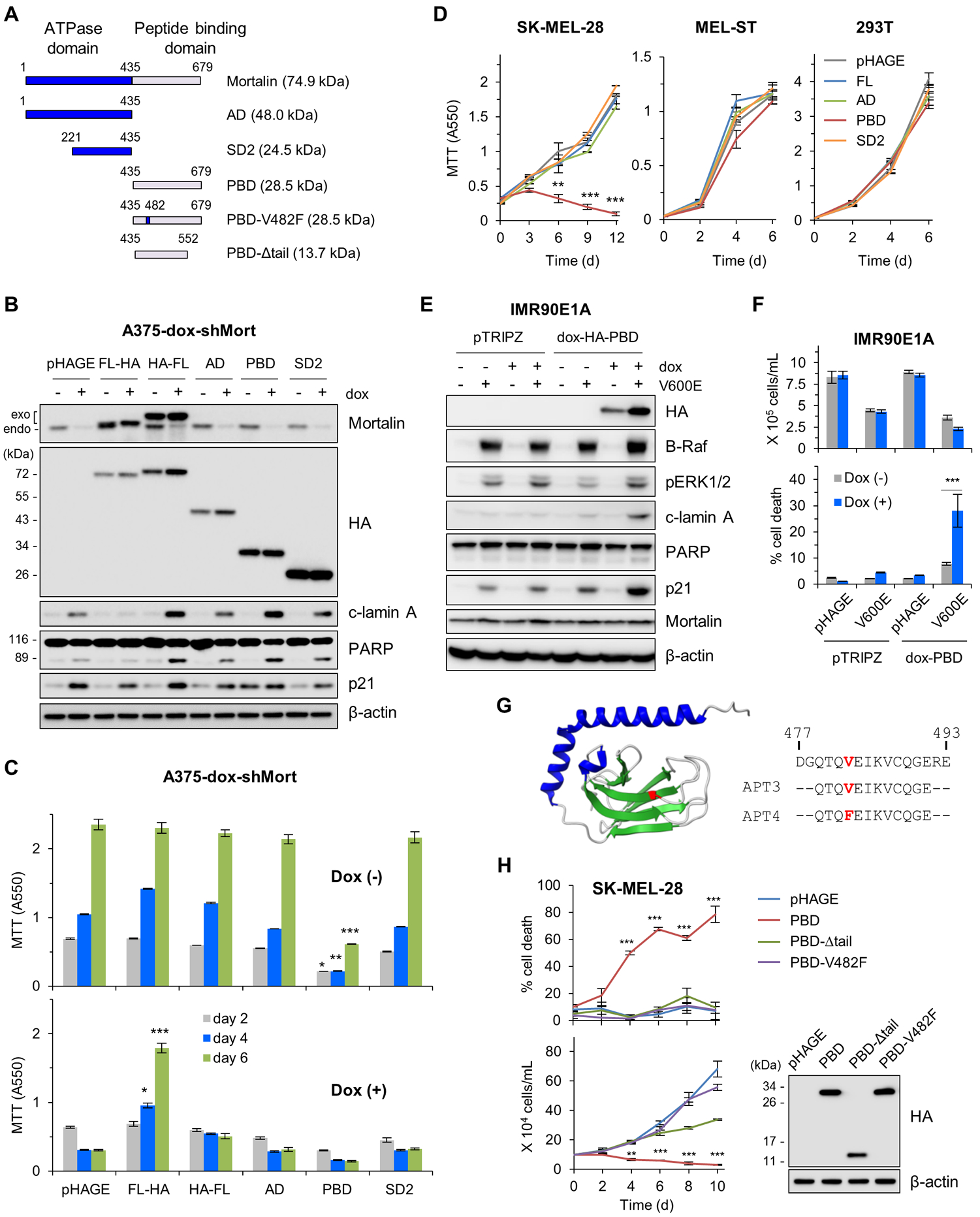
(A) Schematics of mortalin mutants used in this study. AD, ATPase domain; SD2, subdomain 2; PBD, peptide binding domain; V482F, Val482Phe; Δtail, tail deletion. (B and C) A375-dox-shMort cells infected with pHAGE expressing full-length mortalin (FL) or domain mutants were treated with 0.5 μg/ml doxycycline (dox) for 4 days prior to Western blotting of total cell lysates (B) and MTT assay (C). Exogenous and endogenous mortalin proteins are indicated. Densitometry of lamin A and PARP cleavage is presented in fig. S6. (D) MTT assay of cells expressing the indicated mortalin constructs. (E) Western blotting of total cell lysates from IMR90E1A -dox-PBD cells infected with pHAGE-B-RafV600E and treated with 0.5 μg/ml doxycycline for 3 days. pTRIPZ is the empty viral vector control for dox-HA-PBD. (F) Proliferation and death rates of cells in (E) were determined by trypan blue exclusion assays. (G) 3-D structure of mortalin-PBD (PDB:3N8E). Val482 in the substrate-binding cavity is highlighted in red in the structure and in synthetic decoy peptide aptamers (APT) used in this study. (H) Trypan blue exclusion assays of SK-MEL-28 cells expressing PBD mutants. Western blotting of total cell lysates (right panel) shows the expression levels of these constructs. Blots (B, E, and H) are representative of two independent experiments; quantitative data (C, D, F, and H) are mean ± SEM of three biological replicates. *p < 0.05, **p < 0.01, ***p < 0.001 by two-way ANOVA with Bonferroni post-tests.
